Abstract
Mercury (Hg) is an environmental contaminant that can negatively impact the health of humans and wildlife. Albatrosses and large petrels show some of the highest levels of Hg contamination among birds, with potential repercussions for reproduction and survival. Here, body feather total Hg (THg) concentrations were determined in breeding adults of five species of albatrosses and large petrels in the foraging guild at South Georgia during the mid-2010s. We tested the effects of species, sex and trophic ecology (inferred from stable isotopes) on THg concentrations and compared our results with published values from past decades. Feather THg concentrations differed significantly among species (range: 1.9–49.6 µg g−1 dw), and were highest in wandering albatrosses Diomedea exulans, intermediate in black-browed albatrosses Thalassarche melanophris and northern giant petrels Macronectes halli, and lowest in southern giant petrels M. giganteus and white-chinned petrels Procellaria aequinoctialis. Females were more contaminated than males in all species, potentially due to differences in distributions and diet composition. Across species, THg concentrations were not correlated with feather δ13C or δ15N values, implying that species effects (e.g., breeding and moulting frequencies) may be more important than trophic effects in explaining feather THg concentrations in this foraging guild. Within species, the only significant correlation was between THg and δ13C in wandering albatrosses, which could reflect higher Hg exposure in subtropical waters. Comparisons with THg concentrations from past studies, which reflect contamination from 10 to > 60 years ago, revealed considerable annual variation and some evidence for increases over time for wandering and black-browed albatrosses since before 1950 and from the late 1980s, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mercury (Hg) contamination of marine ecosystems is an important environmental issue. Hg entering the environment can be of natural origin (e.g., from volcanism, rock weathering, hydrothermal vents) or anthropogenic, and human inputs have greatly increased the amount of Hg in circulation since the Industrial Revolution (Pirrone et al. 2010; Lamborg et al. 2014; Outridge et al. 2018; Streets et al. 2019). Currently, artisanal and small-scale gold mining (ASGM) is the largest anthropogenic source of Hg released into the atmosphere (Keane et al. 2023). Hg emissions are assumed to be deposited predominantly within the same hemisphere, given that the atmospheric lifetime of 3–6 months is shorter than the timescale for interhemispheric air exchange (Fisher et al. 2023; Schneider et al. 2023). Hg primarily enters the open ocean via atmospheric deposition (Driscoll et al. 2013), and once in this environment, inorganic Hg (iHg) is methylated to organic methyl-Hg (MeHg), principally by microorganisms (Hg methylators), such as iron- and sulfate-reducing bacteria (Hsu-Kim et al. 2013). MeHg, which is the most toxic and bioavailable form of Hg, bioaccumulates within marine organisms, such that concentrations increase in tissues over time, and biomagnifies up marine food chains, increasing from lower to higher trophic levels (Seco et al. 2021). In marine ecosystems, top predators with long lifespans (including large fish, seabirds and marine mammals) can therefore accumulate high Hg concentrations in their tissues (Monteiro and Furness 1995; Chételat et al. 2020).
Feathers are frequently used as a non-destructive means of monitoring Hg contamination of seabirds (Albert et al. 2019). Moult is considered to play a major role in the elimination of MeHg (Braune 1987; Renedo et al. 2021), and Hg bound in the feathers may account for > 90% of the total body burden in some seabirds, though this figure is much lower (< 10%) for albatrosses (Braune and Gaski 1987; Kim et al. 1996). Once bound to the sulfhydryl groups of keratin molecules, Hg cannot be lost from feathers (Crewther et al. 1965; Appelquist et al. 1984). The total Hg concentrations (THg) measured in feathers are almost exclusively MeHg (> 90%) and so are often used as a proxy for MeHg (Renedo et al. 2017). Depending on the seabird species, Hg in the feathers of adults may primarily reflect remobilised Hg that has accumulated in internal tissues between successive moults (i.e., capital strategy) or MeHg derived from recent dietary intake (i.e., income strategy) (Cherel et al. 2018).
Diet analyses can reveal the drivers of contamination levels, as seabirds are mainly exposed to Hg via their prey. Stomach contents or pellets can be used to identify ingested prey to species level, but are biased by the different digestion rates and retention times of prey, and cannot be collected outside of the breeding period if birds are far from the colony (Barrett et al. 2007). Stable isotope analysis offers an alternative approach for relating diet to contaminant burdens, as the isotopic composition of consumer tissues relates predictably to that of their prey (Thompson et al. 1998). Bulk carbon stable isotope values (δ13C) of seabird tissues vary little with trophic level (~ 1‰), but can be used to infer feeding areas (e.g., the relative dependence on inshore vs. offshore, benthic vs. pelagic diets, and on latitude/water mass), whereas those of nitrogen (δ15N) show a stepwise increase with trophic level (~ 3–5‰) (Peterson and Fry 1987; Hobson and Clark 1992; Bearhop et al. 2002; Cherel and Hobson 2007; Phillips et al. 2009). However, baseline δ15N values also vary spatially, which can obscure variation associated with trophic position (Elliott et al. 2021). For instance, high δ13C and δ15N values of feathers from adult seabirds in the southwest Atlantic Ocean indicate that they feed in neritic waters (Phillips et al. 2009; Mills et al. 2024). Stable isotope values of feathers reflect diet during their synthesis, and because they are metabolically inert, they retain this information indefinitely (Cherel et al. 2000). Albatrosses and petrels generally do not breed and moult concurrently (Prince et al. 1993; Cherel et al. 2000; Catry et al. 2013); hence, stable isotope analyses of adult feathers provide dietary information during the nonbreeding period (Cherel et al. 2000; Phillips et al. 2009).
In this study, we investigated the dynamics of Hg contamination among adults of five species of albatrosses and large petrels from the globally important populations at South Georgia, sampled in the mid-2010s. South Georgia is located ~ 300 km south of the Antarctic Polar Front (APF) in the southwest Atlantic Ocean sector of the Southern Ocean. All study species have been tracked in previous years using geolocators (Phillips et al. 2005, 2006; González-Solís et al. 2008; Clay et al. 2018; Granroth-Wilding and Phillips 2019), and we used body feather δ13C and δ15N values to infer feeding areas of individuals in this study during the non-breeding season. Our study species show some of the highest feather THg concentrations among birds; indeed, albatrosses are the most contaminated avian family in terms of Hg (Cherel et al. 2018). Our objectives were to: (i) identify different factors driving feather THg concentrations within this foraging guild (including species, sex and trophic ecology), hypothesising that species characteristics (e.g. breeding and moulting frequencies) are more important than trophic effects (Stewart et al. 1999; Anderson et al. 2009); and (ii) compare contamination levels in the 2010s (measured here), with data from past studies at South Georgia (Thompson et al. 1993; Anderson et al. 2009; Becker et al. 2002, 2016). The latter is especially pertinent as there is evidence for increasing feather THg concentrations of adult grey-headed albatrosses Thalassarche chrysostoma from South Georgia since the late 1980s (Mills et al. 2020a). Moreover, even at low levels, Hg contamination can have a variety of negative effects on seabirds, including on physiology, immune status and behaviour (Tartu et al. 2015; Ibañez et al. 2024), and can ultimately impact breeding success and population dynamics (Mills et al. 2020a; Goutte et al. 2014a, 2014b). Hg contamination during the non-breeding period may also have negative carry-over effects in the subsequent breeding period (Mills et al. 2020a; Carravieri et al. 2023).
Materials and Methods
Study Site, Species and Feather Sampling
Feather sampling of albatrosses and large petrels was undertaken at Bird Island, South Georgia (54°00’S, 38°03’W). South Georgia is a United Kingdom (UK) Overseas Territory at the northern limit of the Scotia Sea. Random samples of body feathers were obtained from breeding adults during the incubation or brood-guard periods of the following species: wandering albatross Diomedea exulans (n = 15), black-browed albatross T. melanophris (n = 15), northern giant petrel Macronectes halli (n = 16), southern giant petrel M. giganteus (n = 16) and white-chinned petrel Procellaria aequinoctialis (n = 12) (Table 1). Southern giant petrels were sampled in the 2011/2012 breeding season and other species in 2014/2015. Body feathers present less variation in Hg levels than flight feathers and their collection does not impair flight performance (Furness et al. 1986). Feathers were stored dry in sealed plastic bags or envelopes and then returned to the British Antarctic Survey (Cambridge, UK) for laboratory analyses. Birds were sexed from records of observed copulatory position, bill sizes or from DNA extracted from blood samples (Fridolfsson and Ellegren 1999). Birds were of unknown age; however, previous studies have not found significant relationships between feather THg concentrations and age in breeding adult albatrosses (Tavares et al. 2013; Bustamante et al. 2016; Mills et al. 2020a).
All study species (excluding the wandering albatross) typically breed annually and during the austral summer, returning to the colony from September to November and fledging chicks from March to June. Wandering albatrosses, however, return from October to November, fledge chicks from November to December in the following year, and breed biennially if successful. Moult and breeding are energetically expensive and do not tend to occur simultaneously in albatrosses and petrels (Prince et al. 1993; Cherel et al. 2000; Catry et al. 2013). However, some body feather moult occurs during the early breeding season in giant petrels, and to a limited extent during the late breeding season in black-browed albatrosses at South Georgia (Hunter 1984; Catry et al. 2013). Growing body feathers, which were present in a few giant petrels, were avoided during sampling. Two generations of body feathers were always apparent, but only the newer, less abraded feathers were collected, hence it is likely that these feathers represent the preceding non-breeding period in all cases. Body feather replacement in the study species occurs gradually during the non-breeding period (~ 7% being moulted and replaced at any one time), and so the exact timing of moult of individual feathers is unknown (Battam et al. 2010).
Total Mercury Analysis
Feathers were cleaned using repeated chloroform:methanol solution (2:1 v/v) and Milli-Q® water rinses. The feather samples were then air-dried under a fume hood for 48 h and cut into very fine fragments using stainless steel scissors. Multiple feathers were pooled and homogenised per individual to ensure compatibility with previous studies at South Georgia (Thompson et al. 1993; Anderson et al. 2009; Becker et al. 2002, 2016). THg concentrations were measured in subsamples of the homogenised body feathers using an Advanced Mercury Analyser spectrophotometer (AMA 254 Altec®) at the laboratory Littoral Environnement et Sociétés (LIENSs, La Rochelle, France). Each sample was analysed in duplicate or triplicate until the relative standard deviation (RSD) between measurements was < 10%. Blanks were analysed at the beginning of each sample run and accuracy was assessed using a certified reference material (CRM), lobster hepatopancreas TORT-3 (National Research Centre [NRC], Canada; certified THg concentration: 0.292 ± 0.022 µg g−1 dw), and our measured concentration was 0.295 ± 0.006 µg g−1 dw (n = 27). Our CRM results were in thus in good agreement with the certified values with a recovery of 101.1 ± 2.1%. The detection limit of the AMA was 0.005 µg g−1 dw. THg concentrations are presented in µg g−1 dw.
Stable Isotope Analysis
Stable isotopes of carbon and nitrogen were measured in the same homogenised feather subsamples as above. Subsamples were weighed (~ 0.3 mg) into 6 × 4 mm tin capsules using a microbalance and stable isotope analyses were undertaken at the Laboratory of Stable Isotopes at the Doñana Biological Station (Seville, Spain). Samples were combusted at 1020 °C with a continuous flow isotope-ratio mass spectrometry system by means of Flash HT Plus elemental analyser coupled to a Delta-V Advantage isotope ratio mass spectrometer via a CONFLO IV interface (ThermoFisher Scientific, Bremen, Germany). Stable isotope ratios are reported using the conventional δ notation (‰) following the equation: δX = [(Rsample/Rstandard) − 1] × 1000, where X is 13C or 15N, R is the corresponding ratio 13C:12C or 15N:14N, and Rstandard is the ratio of international references Vienna Peedee Belemnite for carbon and atmospheric N2 (AIR) for nitrogen. The following internal standards were used: EBD-23 (cow horn), LIE-BB (whale baleen), and LIE-PA (razorbill feathers). Internal standards were routinely inserted into the sampling sequence to correct for linearity and instrument drift. Replicate assays of internal standards indicated analytical precisions of ± 0.1 and ± 0.2‰ for δ13C and δ15N, respectively. Internal standards were calibrated with international standards from the International Atomic Energy Agency (IAEA, Vienna).
Data Analysis
Data were analysed using R version 4.0.3. and visualised using the ggplot2 package (Wickham 2016; R Core Team 2020). Assumptions of normality of residuals and homogeneity of variances were tested using Shapiro–Wilk and Levene’s tests, respectively. THg concentrations were subsequently log-transformed. The effects of species, sex and their two-way interaction on feather THg concentrations were tested using a two-way ANOVA followed by post-hoc Tukey’s HSD tests. The effect of species on feather δ13C and δ15N values was testing using Kruskal–Wallis tests and post-hoc Dunn’s tests. Welch’s t-tests were used to assess sex differences in feather δ13C and δ15N values for each species. Spearman’s rank correlations were used to test for associations between feather THg concentrations and δ13C and δ15N values across species and for each species separately, as pooling species that migrate to distinct habitats with different isotopic baselines (e.g., oceanic vs. continental shelf/shelf-slope waters) may obscure relationships with THg (Anderson et al. 2009; Blévin et al. 2013). Lastly, we compared our data with previously published feather THg concentrations for the study species at South Georgia (Thompson et al. 1993; Anderson et al. 2009; Becker et al. 2002, 2016). Only means, SDs and sample sizes were available from these previous studies (Table 2). Statistical significance was assumed at α = 0.05 in all cases.
Results
Total Hg Concentrations
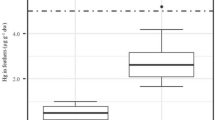
There were significant effects of species and sex on (log-transformed) feather THg concentrations (two-way ANOVA, F4,64 = 43.1, p < 0.001 and F1,64 = 12.4, p < 0.001, respectively). The interaction term was not significant (F4,64 = 0.13, p = 0.97). The general pattern from the post-hoc Tukey’s HSD tests was that feather THg concentrations were considerably higher in wandering albatrosses than other species, intermediate in black-browed albatrosses and northern giant petrels, and lowest in southern giant petrels and white-chinned petrels (Fig. 1). Females had higher THg concentrations than males in all species (Table 1). In a comparison with previous studies, feather THg concentrations of all study species showed annual variation, and there was some evidence for increases over time for wandering and black-browed albatrosses since before 1950 and from the late 1980s, respectively (Table 2).
Boxplots of total Hg concentrations (µg g−1 dw) in body feathers of albatrosses and petrels sampled at Bird Island, South Georgia. Species abbreviations are as follows: BBA = black-browed albatross Thalassarche melanophris; NGP = northern giant petrel Macronectes halli; SGP = southern giant petrel M. giganteus; WA = wandering albatross Diomedea exulans; WCP = white-chinned petrel Procellaria aequinoctialis. Samples were collected from southern giant petrels in the 2011/2012 breeding season and from all other species in 2014/2015. Species sharing superscript letters are not significantly different according to post-hoc Tukey’s HSD tests. Boxplots show medians (horizontal lines), interquartile range (IQR; boxes), the lowest and highest values within 1.5 × IQR (whiskers) and outliers (black points)
Stable Isotopes
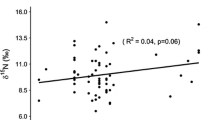
Species had a significant effect on feather δ13C and δ15N values (Kruskal–Wallis tests, χ24 = 55.9, p < 0.0001 and χ24 = 45.5, p < 0.0001, respectively) (Figs. 2, 3). Post-hoc Dunn’s tests showed that the ranking of species in order of increasing (i.e., less negative) δ13C values was southern giant petrel, northern giant petrel, wandering albatross, white-chinned petrel and black-browed albatross (Fig. 3), and of increasing δ15N values was southern giant petrel, northern giant petrel, wandering albatross, black-browed albatross and white-chinned petrel (Fig. 3). Feather δ13C values of female wandering albatrosses were significantly less negative than those of males (Welch’s t-test, t = 2.63, p < 0.05), but there were no other significant differences in feather δ13C and δ15N values for any study species (all p ≥ 0.15) (Table 1). Feather THg concentrations were not significantly correlated with δ13C or δ15N values across species (Spearman’s rank correlations, rho = 0.12, p = 0.30 and rho = 0.05, p = 0.68) (Fig. 4). The only significant correlation within species was between THg and δ13C values in wandering albatrosses (rho = 0.68, p < 0.01) (Fig. 4).
Mean (± SDs) and individual carbon (δ13C) and nitrogen (δ.15N) stable isotope values (‰) of body feathers of albatrosses and petrels sampled at Bird Island, South Georgia. Species abbreviations are: BBA = black-browed albatross Thalassarche melanophris; NGP = northern giant petrel Macronectes halli; SGP = southern giant petrel M. giganteus; WA = wandering albatross Diomedea exulans; WCP = white-chinned petrel Procellaria aequinoctialis. Samples were collected from southern giant petrels in the 2011/2012 breeding season and from all other species in 2014/2015. Grey vertical shaded areas and text reflect the approximate locations of biogeographic boundary zones (Phillips et al. 2009)
Boxplots of (a) carbon (δ13C) and (b) nitrogen (δ15N) stable isotope values (‰) in body feathers of albatrosses and large petrels sampled at Bird Island, South Georgia. Species abbreviations are: BBA = black-browed albatross Thalassarche melanophris; NGP = northern giant petrel Macronectes halli; SGP = southern giant petrel M. giganteus; WA = wandering albatross Diomedea exulans; WCP = white-chinned petrel Procellaria aequinoctialis. Samples were collected from southern giant petrels in the 2011/2012 breeding season and from all other species in 2014/2015. Species sharing superscript letters are not significantly different according to post-hoc Dunn’s tests. Boxplots show medians (horizontal lines), interquartile range (IQR; boxes), the lowest and highest values within 1.5 × IQR (whiskers) and outliers (black points)
Relationships between body feather total Hg (THg) concentrations (µg g−1 dw) and (a) δ13C and (b) δ15N values (‰) of body feathers of albatrosses and large petrels; and (c) relationships between feather THg concentrations and δ13C values of wandering albatross Diomedea exulans sampled at Bird Island, South Georgia. Species abbreviations are: BBA = black-browed albatross Thalassarche melanophris; NGP = northern giant petrel Macronectes halli; SGP = southern giant petrel M. giganteus; WA = wandering albatross; WCP = white-chinned petrel Procellaria aequinoctialis
Discussion
Certain life-history and ecological traits of albatrosses and large petrels (e.g., high trophic positions) lead them to accumulate high Hg concentrations in their tissues. In this study, we measured body feather THg concentrations of five species in the foraging guild of albatrosses and large petrels at South Georgia during the mid-2010s, where there are globally important breeding populations of all study species. Our study analysed the underlying drivers of variation in Hg contamination and compared the THg concentrations with those of previous studies, reflecting contamination during the previous two to over six decades.
Interspecific Differences in Hg Contamination
Species had a significant effect on feather THg concentrations in our study. Most notably, feather THg concentrations of wandering albatrosses were far higher than our other study species (Fig. 1). THg concentrations were also higher than those of wandering albatrosses at the Crozet (mean ± SD, 22.14 ± 10.30 µg g−1 dw), Kerguelen (16.59 ± 3.78 µg g−1 dw) and Prince Edward Islands (24.83 ± 12.35 µg g−1 dw) (Thompson et al. 1993; Carravieri et al. 2014a; Bustamante et al. 2016; Cherel et al. 2018), and of grey-headed albatrosses sampled at Bird Island in 2013/14 (13.08 ± 6.56 µg g−1 dw) (Mills et al. 2020a). Among albatrosses and petrels, only the Amsterdam albatross D. amsterdamensis has higher mean feather THg concentrations (34.60 ± 12.50 µg g−1 dw) (Cherel et al. 2018).
Different moulting patterns provide one explanation for the significant interspecific differences in THg concentrations in our study, as feather THg concentrations do not just reflect exposure during synthesis (i.e., dietary intake), but also the release of Hg accumulated since the previous moult (Anderson et al. 2009; Carravieri et al. 2014b; Cherel et al. 2018). Species that take several years to moult all their feathers will accumulate Hg over a considerably longer period than those which replace all feathers annually (Stewart et al. 1999; Anderson et al. 2009). Although this seems likely to partly explains the higher Hg concentrations of wandering albatrosses compared to our other study species, which is the only biennial breeder in our study, breeding frequency (i.e., annual vs. biennial) was not an explanatory factor in models of feather THg concentrations of various albatross species in the review by Cherel et al. (2018), and feather concentrations were considered to predominantly reflect dietary intake.
An alternative explanation for the significant interspecific differences in THg concentrations is variation in diets and feeding areas, and hence dietary exposure to MeHg. However, the interspecific pattern of feather δ13C values did not correspond exactly with THg concentrations (Figs. 1 and 2; see below), which is likely because the different species migrate to habitats with different isotopic baselines in the Southern Ocean (Anderson et al. 2009). There is a general pattern of increasing Hg contamination of seabirds feeding in subtropical compared to subantarctic and Antarctic waters in the Southern Ocean (Renedo et al. 2020), and from species that feed in coastal to more oceanic waters (Ochoa-Acuna et al. 2002). Wandering albatrosses forage in various habitats during the non-breeding period, though mostly in subantarctic to subtropical waters within the southwest Atlantic Ocean (Fig. 2) (Phillips et al. 2009; Clay et al. 2018). Hence, their use of lower latitude foraging areas likely contributes to their high Hg contamination levels. In contrast, as reflected in our isotope data, most southern giant petrels, which showed much lower THg concentrations, remain in waters south of the APF during the non-breeding season (Fig. 2). This corresponds with previous tracking studies, which show that southern giant petrels exploit Antarctic waters to a greater extent than northern giant petrels, many of which utilise the Patagonian Shelf or subantarctic waters to the north of South Georgia (Phillips et al. 2009; González-Solís et al. 2008; Granroth-Wilding and Phillips 2019). The high feather δ13C and δ15N values of white-chinned petrels and black-browed albatrosses are indicative of feeding in continental shelf and shelf-slope regions (Fig. 2) (Phillips et al. 2009; Mills et al. 2024), with the former feeding on the Patagonian Shelf and Humboldt Upwelling System off Chile during the non-breeding period (Phillips et al. 2006), and the latter migrating to the Benguela Upwelling System off southwest Africa (Phillips et al. 2005). Many of the birds that migrate to continental shelf and shelf-slope waters will follow vessels, feeding on discarded demersal fishes which potentially have a high Hg content, possibly because they have longer lifespans and feed at higher trophic levels on prey that usually have higher Hg levels than those in the epipelagic zone (Arcos et al. 2002; Petersen et al. 2008). Our stable isotope data indicate that these descriptions of non-breeding distributions are appropriate for the individuals in our study (Fig. 2). Diet composition, including the consumption of prey from different depths in the water column or of different sizes, may also influence Hg exposure. However, conventional diet data are not available for our study species during the non-breeding period, when they are far from land and are not accessible for sampling.
Sex Differences in Hg Contamination
There was a significant effect of sex on feather THg concentrations in our study, with females having higher levels of Hg contamination than males even though some Hg in females may be deposited in the egg (Robinson et al. 2012; Carravieri et al. 2014a). The sex-species interaction was not significant. Assuming a strong influence of dietary intake on feather THg of albatrosses and giant petrels (Cherel et al. 2018; Renedo et al. 2021), differences in contamination may be due to sex-specific differences in diets and feeding areas (Carravieri et al. 2014a; Bustamante et al. 2016). Indeed, higher feather and blood THg concentrations of female compared to male wandering albatrosses at the Crozet Islands were attributed to greater time spent foraging in subtropical and subantarctic waters than in Antarctic waters (Carravieri et al. 2014a; Bustamante et al. 2016). Our study found that feather δ13C values were more negative in male than female wandering albatrosses, potentially indicating higher-latitude feeding areas. A previous study at South Georgia found a weak overall effect of sex on δ13C in the sexually size-dimorphic albatrosses and giant petrels, although none of the comparisons within species were statistically significant (Phillips et al. 2009). Tracking studies in the Indian Ocean indicate there is some sexual segregation by latitude in core foraging areas of non-breeding wandering albatrosses, with females tending to feed in more northerly waters (Weimerskirch et al. 2014). Geolocator data from non-breeding black-browed albatrosses show that females feed 4 to 5° further north within the Benguela Upwelling system than males (Phillips et al. 2005); however, there is a degree of overlap which may be why there was no difference between sexes in mean feather δ13C values. There is limited evidence for sexual segregation in foraging areas of northern giant petrels during winter, although males have a larger foraging range than females; however, female southern giant petrels forage more on the southern Patagonian shelf-break and males are mostly restricted to South Georgia and more southerly waters (González-Solís et al. 2008). Hence it appears that the sex differences in feather Hg concentrations in some study species may result from differences in diet composition or other aspects of foraging behaviour or distribution that are too subtle to be reflected in the stable isotope data. Small sample sizes of some species or sex groups may have also contributed to the lack of significant differences.
Influence of Diets and Distributions
THg concentrations were not correlated with feather δ13C or δ15N values across species (see above). The wandering albatross was the only species for which we found a significant correlation between THg concentrations and stable isotope values (Fig. 4). The relatively weak correlation with δ13C values may be because we analysed pooled rather than individual feathers, to ensure comparability with previous data (Cherel et al. 2018). Additionally, integration periods for stable isotopes and THg into feathers will differ if MeHg is maintained in a body reservoir until it can be eliminated (Bond 2010). However, THg in body feathers of albatrosses appears to be a faithful reflection of Hg exposure on moulting grounds (Cherel et al. 2018). In the Southern Ocean, feather δ13C values of wandering albatrosses should principally reflect foraging latitude, with values increasing from waters in Antarctica towards the subantarctic and subtropics (Cherel and Hobson 2007; Phillips et al. 2009). Although two studies found that MeHg concentrations were higher in waters to the south than north of the APF (Cossa et al. 2011; Yue et al. 2023), there is a well-documented pattern of increasing Hg contamination of seabirds feeding in Antarctic and subantarctic waters compared to those in the subtropics (Carravieri et al. 2016, 2017; Renedo et al. 2020; Mills et al. 2022) This may reflect spatial differences in the bioavailability of Hg to seabirds, potentially due to greater vertical mixing and more efficient Hg methylation at depth in lower latitudes (Renedo et al. 2020). Differences in food chain lengths with latitude could also contribute to these spatial differences, which are potentially shorter at higher than at lower latitudes (Forero et al. 2005; Renedo et al. 2020). There were no significant correlations between THg concentrations and δ15N values for any study species. Typically, δ15N is used as a proxy for trophic level, and therefore values are expected to correlate with THg because of biomagnification through the food web. However, moulting habitats of our study species are likely marked by variable δ15N baselines (St John Glew et al. 2021). Hence δ15N values across the study species may not relate directly to trophic level. Future studies at South Georgia could use compound-specific stable isotope analyses of amino acids to provide unbiased estimates of trophic positions (Elliott et al. 2021), as has been conducted on some seabird species elsewhere in the Southern Ocean (Thébault et al. 2021; Quillfeldt et al. 2023).
Long-Term Changes in Hg Contamination
Data on feather THg concentrations were available from the late 1990s and early 2000s for giant petrels and white-chinned petrels, the late 1980s and the mid 2000s for black-browed albatrosses, and before 1950, the late 1980s and mid 2000s for wandering albatrosses (Table 2). However, caution should be applied when comparing data among previous studies as the methods used to quantify THg concentrations differ. For instance, one study extracted organic Hg to overcome the application of iHg as a preservative on museum specimens (Thompson et al. 1993). Distinguishing typical annual variation from genuine long-term trends is challenging because of the limited availability of previous data; nevertheless, there was some evidence for a slight increase over time in feather THg concentrations of black-browed albatrosses and wandering albatrosses since the late 1980s and before 1950, respectively (Table 2). However, annual variation was high, and trends were less convincing than for increasing Hg contamination of grey-headed albatrosses at South Georgia since the late 1980s (Mills et al. 2020a). Increasing Hg contamination could indicate increased environmental exposure within feeding areas (i.e., changes in bioavailability of MeHg within foraging areas), potentially due to increasing anthropogenic Hg emissions in the Southern Hemisphere across our study period (Streets et al. 2017). Shifts in diets and foraging areas towards more contaminated prey or regions could also explain temporal variation. Diet composition of albatrosses and petrels can be highly variable among years, at least during the breeding season (Mills et al. 2020b, 2021). Quantifying diets and foraging areas alongside levels of Hg contamination can help interpret trends, but stable isotope data were not available in all previous studies (Table 2). More work is required to understand long-term drivers and trends in contamination of seabirds in general, including the relative importance of natural and anthropogenic changes in the environment. Museum specimens may be a useful source of material for extending time series for some species and sites.
Data Availability
The data supporting this study will be made available from the corresponding author upon reasonable request.
References
Albert C, Renedo M, Bustamante P, Fort J (2019) Using blood and feathers to investigate large-scale Hg contamination in Arctic seabirds: a review. Environ Res 177:108588. https://doi.org/10.1016/j.envres.2019.108588
Anderson ORJ, Phillips RA, McDonald RA, Shore RF, McGill RAR, Bearhop S (2009) Influence of trophic position and foraging range on mercury levels within a seabird community. Mar Ecol Prog Ser 375:277–288. https://doi.org/10.3354/meps07784
Appelquist H, Asbirk S, Drabæk I (1984) Mercury monitoring: mercury stability in bird feathers. Mar Pollut Bull 15:22–24. https://doi.org/10.1016/0025-326X(84)90419-3
Arcos JM, Ruiz X, Bearhop S, Furness RW (2002) Mercury levels in seabirds and their fish prey at the Ebro Delta (NW Mediterranean): the role of trawler discards as a source of contamination. Mar Ecol Prog Ser 232:281–290. https://doi.org/10.3354/meps232281
Barrett RT, Camphuysen K, Anker-Nilssen T, Chardine JW, Furness RW, Garthe S, Hüppop O, Leopold MF, Montevecchi WA, Veit RR (2007) Diet studies of seabirds: a review and recommendations. ICES J Mar Sci 64:1675–1691. https://doi.org/10.1093/icesjms/fsm152
Battam H, Richardson M, Watson AWT, Buttemer WA (2010) Chemical composition and tissue energy density of the cuttlefish (Sepia apama) and its assimilation efficiency by Diomedea albatrosses. J Comp Physiol B 180:1247–1255. https://doi.org/10.1007/s00360-010-0497-3
Bearhop S, Waldron S, Votier SC, Furness RW (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol Biochem Zool 75:451–458. https://doi.org/10.1086/342800
Becker PH, González-Solís J, Behrends B, Croxall J (2002) Feather mercury levels in seabirds at South Georgia: influence of trophic position, sex and age. Mar Ecol Prog Ser 243:261–269. https://doi.org/10.3354/meps243261
Becker PH, Goutner V, Ryan PG, González-Solís J (2016) Feather mercury concentrations in Southern Ocean seabirds: variation by species, site and time. Environ Pollut 216:253–263. https://doi.org/10.1016/j.envpol.2016.05.061
Blévin P, Carravieri A, Jaeger A, Chastel O, Bustamante P, Cherel Y (2013) Wide range of mercury contamination in chicks of Southern Ocean Seabirds. PLoS ONE 8:e54508. https://doi.org/10.1371/journal.pone.0054508
Bond AL (2010) Relationships between stable isotopes and metal contaminants in feathers are spurious and biologically uninformative. Environ Pollut 158:1182–1184. https://doi.org/10.1016/j.envpol.2010.01.004
Braune BM (1987) Comparison of total mercury levels in relation to diet and molt for nine species of marine birds. Arch Environ Contam Toxicol 16:217–224. https://doi.org/10.1007/BF01055802
Braune BM, Gaskin DE (1987) Mercury levels in Bonaparte’s gulls (Larus Philadelphia) during autumn molt in the Quoddy region, New Brunswick, Canada. Arch Environ Contam Toxicol 16:539–549. https://doi.org/10.1007/BF01055810
Bustamante P, Carravieri A, Goutte A, Barbraud C, Delord K, Chastel O, Weimerskirch H, Cherel Y (2016) High feather mercury concentrations in the wandering albatross are related to sex, breeding status and trophic ecology with no demographic consequences. Environ Res 144:1–10. https://doi.org/10.1016/j.envres.2015.10.024
Carravieri A, Bustamante P, Tartu S, Meillère A, Labadie P, Budzinski H, Peluhet L, Barbraud C, Weimerskirch H, Chastel O, Cherel Y (2014a) Wandering albatrosses document latitudinal variations in the transfer of persistent organic pollutants and mercury to Southern Ocean predators. Environ Sci Technol 48:14746–14755. https://doi.org/10.1021/es504601m
Carravieri A, Cherel Y, Blévin P, Brault-Favrou M, Chastel O, Bustamante P (2014b) Mercury exposure in a large subantarctic avian community. Environ Pollut 190:51–57. https://doi.org/10.1016/j.envpol.2014.03.017
Carravieri A, Cherel Y, Jaeger A, Churlaud C, Bustamante P (2016) Penguins as bioindicators of mercury contamination in the southern Indian Ocean: geographical and temporal trends. Environ Pollut 213:195–205. https://doi.org/10.1016/j.envpol.2016.02.010
Carravieri A, Cherel Y, Brault-Favrou M, Churlaud C, Pehluet L, Labadie P, Budzinski H, Chastel O, Bustamante P (2017) From Antarctica to the subtropics: contrasted geographical concentrations of selenium, mercury, and persistent organic pollutants in skua chicks (Catharacta spp.). Environ Pollut 228:464–473. https://doi.org/10.1016/j.envpol.2017.05.053
Carravieri A, Lorioux S, Angelier F, Chastel O, Albert C, Bråthen VS, Brisson-Curadeau É, Clairbaux M, Delord K, Giraudeau M, Perret S (2023) Carryover effects of winter mercury contamination on summer concentrations and reproductive performance in little auks. Environ Pollut 318:120774. https://doi.org/10.1016/j.envpol.2022.120774
Catry P, Poisbleau M, Lecoq M, Phillips RA (2013) Differences in the timing and extent of annual moult of black-browed albatrosses Thalassarche melanophris living in contrasting environments. Polar Biol 36:837–842. https://doi.org/10.1007/s00300-013-1309-5
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287. https://doi.org/10.3354/meps329281
Cherel Y, Hobson KA, Weimerskirch H (2000) Using stable-isotope analysis of feathers to distinguish moulting and breeding origins of seabirds. Oecologia 122:155–162. https://doi.org/10.1007/PL00008843
Cherel Y, Barbraud C, Lahournat M, Jaeger A, Jaquemet S, Wanless RM, Phillips RA, Thompson DR, Bustamante P (2018) Accumulate or eliminate? Seasonal mercury dynamics in albatrosses, the most contaminated family of birds. Environ Pollut 241:124–135. https://doi.org/10.1016/j.envpol.2018.05.048
Chételat J, Ackerman JT, Eagles-Smith CA, Hebert CE (2020) Methylmercury exposure in wildlife: a review of the ecological and physiological processes affecting contaminant concentrations and their interpretation. Sci Total Environ 711:135117. https://doi.org/10.1016/j.scitotenv.2019.135117
Clay TA, Pearmain EJ, McGill RAR, Manica A, Phillips RA (2018) Age-related variation in non-breeding foraging behaviour and carry-over effects on fitness in an extremely long-lived bird. Funct Ecol 32:1832–1846. https://doi.org/10.1111/1365-2435.13120
Cossa D, Heimbürger LE, Lannuzel D, Rintoul SR, Butler EC, Bowie AR, Averty B, Watson RJ, Remenyi T (2011) Mercury in the Southern Ocean. Geochim Cosmochim Acta 75:4037–4052. https://doi.org/10.1016/j.gca.2011.05.001
Crewther WG, Fraser RDB, Lennox FG, Lindley H (1965) The chemistry of keratins. Adv Protein Chem 20:191–346. https://doi.org/10.1016/S0065-3233(08)60390-3
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Tech 47:4967–4983. https://doi.org/10.1021/es305071v
Elliott KH, Braune BM, Elliott JE (2021) Beyond bulk δ15N: combining a suite of stable isotopic measures improves the resolution of the food webs mediating contaminant signals across space, time and communities. Environ Int 148:106370. https://doi.org/10.1016/j.envint.2020.106370
Fisher JA, Schneider L, Fostier AH, Guerrero S, Guimarães JRD, Labuschagne C, Leaner JJ, Martin LG, Mason RP, Somerset V, Walters C (2023) A synthesis of mercury research in the Southern Hemisphere, part 2: anthropogenic perturbations. Ambio 52:918–937. https://doi.org/10.1007/s13280-023-01840-5
Forero MG, González-Solís J, Hobson KA, Donázar JA, Bertellotti M, Blanco G, Bortolotti GR (2005) Stable isotopes reveal trophic segregation by sex and age in the southern giant petrel in two different food webs. Mar Ecol Prog Ser 296:107–113. https://doi.org/10.3354/meps296107
Fridolfsson A-K, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116. https://doi.org/10.2307/3677252
Furness RW, Muirhead SJ, Woodburn M (1986) Using bird feathers to measure mercury in the environment: relationships between mercury content and moult. Mar Pollut Bull 17:27–30. https://doi.org/10.1016/0025-326X(86)90801-5
González-Solís J, Croxall JP, Afanasyev V (2008) Offshore spatial segregation in giant petrels Macronectes spp.: differences between species, sexes and seasons. Aquat Conserv 17:S22–S36. https://doi.org/10.1002/aqc.911
Goutte A, Barbraud C, Meillère A, Carravieri A, Bustamante P, Labadie P, Budzinski H, Delord K, Cherel Y, Weimerskirch H, Chastel O (2014a) Demographic consequences of heavy metals and persistent organic pollutants in a vulnerable long-lived bird, the wandering albatross. Proc R Soc B 281:20133313. https://doi.org/10.1098/rspb.2013.3313
Goutte A, Bustamante P, Barbraud C, Delord K, Weimerskirch H, Chastel O (2014b) Demographic responses to mercury exposure in two closely-related Antarctic top predators. Ecology 95:1075–1086. https://doi.org/10.1890/13-1229.1
Granroth-Wilding HMV, Phillips RA (2019) Segregation in space and time explains the coexistence of two sympatric sub-Antarctic petrels. Ibis 161:101–116. https://doi.org/10.1111/ibi.12584
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 94:181–188. https://doi.org/10.2307/1368807
Hsu-Kim H, Kucharzyk KH, Zhang T, Deshusses MA (2013) Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review. Environ Sci Tech 47:2441–2456. https://doi.org/10.1021/es304370g
Hunter S (1984) Moult of the giant petrels Macronectes halli and M. giganteus at South Georgia. Ibis 126:119–132. https://doi.org/10.1111/j.1474-919X.1984.tb07993.x
Ibañez AE, Mills WF, Bustamante P, Morales LM, Torres DS, d’Astek B, Mariano-Jelicich R, Phillips RA, Montalti D (2024) Deleterious effects of mercury contamination on immunocompetence, liver function and egg volume in an antarctic seabird. Chemosphere 346:140630. https://doi.org/10.1016/j.chemosphere.2023.140630
Keane S, Bernaudat L, Davis KJ, Stylo M, Mutemeri N, Singo P, Twala P, Mutemeri I, Nakafeero A, Etui ID (2023) Mercury and artisanal and small-scale gold mining: review of global use estimates and considerations for promoting mercury-free alternatives. Ambio 52:833–852. https://doi.org/10.1007/s13280-023-01843-2
Kim EY, Murakami T, Saeki K, Tatsukawa R (1996) Mercury levels and its chemical form in tissues and organs of seabirds. Arch Environ Contam Toxicol 30:259–266. https://doi.org/10.1007/BF00215806
Lamborg CH, Hammerschmidt CR, Bowman KL, Swarr GJ, Munson KM, Ohnemus DC, Lam PJ, Heimbürger LE, Rijkenberg MJ, Saito MA (2014) A global ocean inventory of anthropogenic mercury based on water column measurements. Nature 512:65–68. https://doi.org/10.1038/nature13563
Mills WF, Bustamante P, McGill RAR, Anderson ORJ, Bearhop S, Cherel Y, Votier SC, Phillips RA (2020a) Mercury exposure in an endangered seabird: long-term changes and relationships with trophic ecology and breeding success. Proc R Soc B 287:20202683. https://doi.org/10.1098/rspb.2020.2683
Mills WF, Xavier JC, Bearhop S, Cherel Y, Votier SC, Waluda CM, Phillips RA (2020b) Long-term trends in albatross diets in relation to prey availability and breeding success. Mar Biol 167:29. https://doi.org/10.1007/s00227-019-3630-1
Mills WF, Morley TI, Votier SC, Phillips RA (2021) Long-term inter- and intraspecific dietary variation in sibling seabird species. Mar Biol 168:31. https://doi.org/10.1007/s00227-021-03839-6
Mills WF, Ibañez AE, Bustamante P, Carneiro APB, Bearhop S, Cherel Y, Mariano-Jelicich R, McGill RAR, Montalti D, Votier SC, Phillips RA (2022) Spatial and sex differences in mercury contamination of skuas in the Southern Ocean. Environ Pollut 297:118841. https://doi.org/10.1016/j.envpol.2022.118841
Mills WF, Ibañez AE, Carneiro APB, Morales LM, Mariano-Jelicich R, McGill RAR, Montalti D, Phillips RA (2024) Migration strategies of skuas in the southwest Atlantic Ocean revealed by stable isotopes. Mar Biol 171:27. https://doi.org/10.1007/s00227-023-04347-5
Monteiro LR, Furness RW (1995) Seabirds as monitors of mercury in the marine environment. Water Air Soil Pollut 80:851–870. https://doi.org/10.1007/BF01189736
Ochoa-Acuna H, Sepúlveda MS, Gross TS (2002) Mercury in feathers from Chilean birds: influence of location, feeding strategy, and taxonomic affiliation. Mar Pollut Bull 44:340–345. https://doi.org/10.1016/S0025-326X(01)00280-6
Outridge PM, Mason RP, Wang F, Guerrero S, Heimbürger-Boavida LE (2018) Updated global and oceanic mercury budgets for the United Nations Global Mercury Assessment 2018. Environ Sci Technol 52:11466–11477. https://doi.org/10.1021/acs.est.8b01246
Petersen SL, Phillips RA, Ryan PG, Underhill LG (2008) Albatross overlap with fisheries in the Benguela Upwelling System: implications for conservation and management. Endanger Species Res 5:117–127. https://doi.org/10.3354/esr00132
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320. https://doi.org/10.1146/annurev.es.18.110187.001453
Phillips RA, Silk JRD, Croxall JP, Afanasyev V, Bennett VJ (2005) Summer distribution and migration of nonbreeding albatrosses: individual consistencies and implications for conservation. Ecology 86:2386–2396. https://doi.org/10.1890/04-1885
Phillips RA, Silk JRD, Croxall JP, Afanasyev V (2006) Year-round distribution of white-chinned petrels from South Georgia: Relationships with oceanography and fisheries. Biol Conserv 129:336–347. https://doi.org/10.1016/j.biocon.2005.10.046
Phillips RA, Bearhop S, McGill RAR, Dawson DA (2009) Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 160:795–806. https://doi.org/10.1007/s00442-009-1342-9
Pirrone N, Cinnirella S, Feng X, Finkelman RB, Friedli HR, Leaner J, Mason R, Mukherjee AB, Stracher GB, Streets DG, Telmer K (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos Chem Phys 10:5951–5964. https://doi.org/10.5194/acp-10-5951-2010
Prince PA, Rodwell S, Jones M, Rothery P (1993) Moult in black-browed and grey-headed albatrosses Diomedea melanophris and D. chrysostoma. Ibis 135:121–131. https://doi.org/10.1111/j.1474-919X.1993.tb02823.x
Quillfeldt P, Bedolla-Guzmán Y, Libertelli MM, Cherel Y, Massaro M, Bustamante P (2023) Mercury in ten storm-petrel populations from the Antarctic to the Subtropics. Arch Environ Contam Toxicol 85:55–72. https://doi.org/10.1007/s00244-023-01011-3
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Renedo M, Bustamante P, Tessier E, Pedrero Z, Cherel Y, Amouroux D (2017) Assessment of mercury speciation in feathers using species-specific isotope dilution analysis. Talanta 174:100–110. https://doi.org/10.1016/j.talanta.2017.05.081
Renedo M, Bustamante P, Cherel Y, Pedrero Z, Tessier E, Amouroux D (2020) A “seabird-eye” on mercury stable isotopes and cycling in the Southern Ocean. Sci Total Environ 742:140499. https://doi.org/10.1016/j.scitotenv.2020.140499
Renedo M, Pedrero Z, Amouroux D, Cherel Y, Bustamante P (2021) Mercury isotopes of key tissues document mercury metabolic processes in seabirds. Chemosphere 263:127777. https://doi.org/10.1016/j.chemosphere.2020.127777
Robinson SA, Lajeunesse MJ, Forbes MR (2012) Sex differences in mercury contamination of birds: testing multiple hypotheses with meta-analysis. Environ Sci Technol 46:7094–7101. https://doi.org/10.1021/es204032m
Schneider L, Fisher JA, Diéguez MC, Fostier AH, Guimarães JRD, Leaner JJ, Mason R (2023) A synthesis of mercury research in the Southern Hemisphere, part 1: natural processes. Ambio 52:897–917. https://doi.org/10.1007/s13280-023-01832-5
Seco J, Aparício S, Brierley AS, Bustamante P, Ceia FR, Coelho JP, Phillips RA, Saunders RA, Fielding S, Gregory S, Matias RS, Pardal MA, Pereira E, Stowasser G, Tarling GA, Xavier JC (2021) Mercury biomagnification in a Southern Ocean food web. Environ Pollut 275:116620. https://doi.org/10.1016/j.envpol.2021.116620
St John Glew K, Espinasse B, Hunt BP, Pakhomov EA, Bury SJ, Pinkerton M, Nodder SD, Gutiérrez‐Rodríguez A, Safi K, Brown JC, Graham L (2021) Isoscape models of the Southern Ocean: predicting spatial and temporal variability in carbon and nitrogen isotope compositions of particulate organic matter. Global Biogeochem. Cycles 35:e2020GB006901. https://doi.org/10.1029/2020GB006901
Stewart FM, Phillips RA, Bartle JA, Craig J, Shooter D (1999) Influence of phylogeny, diet, moult schedule and sex on heavy metal concentrations in New Zealand Procellariiformes. Mar Ecol Prog Ser 178:295–305. https://doi.org/10.3354/meps178295
Streets DG, Horowitz HM, Jacob DJ, Lu Z, Levin L, ter Schure AFH, Sunderland EM (2017) Total mercury released to the environment by human activities. Environ Sci Technol 51:5969–5977. https://doi.org/10.1021/acs.est.7b00451
Streets DG, Horowitz HM, Lu Z, Levin L, Thackray CP, Sunderland EM (2019) Five hundred years of anthropogenic mercury: spatial and temporal release profiles. Environ Res Lett 14:084004. https://doi.org/10.1088/1748-9326/ab281f
Tartu S, Angelier F, Wingfield JC, Bustamante P, Labadie P, Budzinski H, Weimerskirch H, Bustnes JO, Chastel O (2015) Corticosterone, prolactin and egg neglect behavior in relation to mercury and legacy POPs in a long-lived Antarctic bird. Sci Total Environ 505:180–188. https://doi.org/10.1016/j.scitotenv.2014.10.008
Tavares S, Xavier JC, Phillips RA, Pereira ME, Pardal MA (2013) Influence of age, sex and breeding status on mercury accumulation patterns in the wandering albatross Diomedea exulans. Environ Pollut 181:315–320. https://doi.org/10.1016/j.envpol.2013.06.032
Thébault J, Bustamante P, Massaro M, Taylor G, Quillfeldt P (2021) Influence of species-specific feeding ecology on mercury concentrations in seabirds breeding on the Chatham Islands, New Zealand. Environ Toxicol Chem 40:454–472. https://doi.org/10.1002/etc.4933
Thompson DR, Furness RW, Lewis SA (1993) Temporal and spatial variation in mercury concentrations in some albatrosses and petrels from the sub-Antarctic. Polar Biol 13:239–244. https://doi.org/10.1007/BF00238759
Thompson DR, Bearhop S, Speakman JR, Furness RW (1998) Feathers as a means of monitoring mercury in seabirds: Insights from stable isotope analysis. Environ Pollut 101:193–200. https://doi.org/10.1016/S0269-7491(98)00078-5
Weimerskirch H, Cherel Y, Delord K, Jaeger A, Patrick SC, Riotte-Lambert L (2014) Lifetime foraging patterns of the wandering albatross: Life on the move! J Exp Mar Biol Ecol 450:68–78. https://doi.org/10.1016/j.jembe.2013.10.021
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York
Yue F, Li Y, Zhang Y, Wang L, Li D, Wu P, Liu H, Lin L, Li D, Hu J, Xie Z (2023) Elevated methylmercury in Antarctic surface seawater: The role of phytoplankton mass and sea ice. Sci Total Environ 882:163646. https://doi.org/10.1016/j.scitotenv.2023.163646
Acknowledgements
The authors are grateful to the editor and the two anonymous reviewers for their constructive comments, which greatly improved the manuscript. The authors thank the many fieldworkers at Bird Island who helped with the sampling of feathers from seabirds, and Maud Brault-Favrou and Sarai López for their help with laboratory analyses. The CPER (Contrat de Projet Etat-Région) and FEDER (Fonds Européen de Développement Régional) are acknowledged for funding the AMA of LIENSs laboratory. PB is an honorary member of the IUF (Institut Universitaire de France). FR acknowledges the Spanish government through the ‘Severo Ochoa Centre of Excellence’ accreditation (CEX2019-000928-S, ICM-CSIC), the project SOSPEN (Plan Estatal de Investigación Científica, Técnica y de Innovación, 2021, PID2021-124831OA-I00), and the Ramón y Cajal programm (Spanish Ministerio de Ciencia e Innovación, RYC2020-030078-I). This work represents a contribution to the Ecosystems component of the British Antarctic Survey Polar Science for Planet Earth Programme, funded by NERC.
Funding
This research was supported by the Natural Environment Research Council (NERC) National Capability Science: Strategic Research & Innovation Short Projects. WFM was awarded a travel grant from the Royal Society of Biology to visit the Laboratory of Stable Isotopes at the Doñana Biological Station.
Author information
Authors and Affiliations
Contributions
William F. Mills: Conceptualization, Methodology, Investigation, Formal Analysis, Visualization, Writing—Original Draft. Paco Bustamante: Investigation, Writing—Review & Editing. Francisco Ramírez: Investigation, Writing—Review & Editing. Manuela G. Forero: Investigation, Writing—Review & Editing. Richard A. Phillips: Conceptualization, Resources, Writing—Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Statement
Feather sampling was approved by the British Antarctic Survey Animal Welfare and Ethics Review Body and carried out under permit from the Government of South Georgia and the South Sandwich Islands.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mills, W.F., Bustamante, P., Ramírez, F. et al. Mercury Concentrations in Feathers of Albatrosses and Large Petrels at South Georgia: Contemporary Patterns and Comparisons with Past Decades. Arch Environ Contam Toxicol 86, 363–374 (2024). https://doi.org/10.1007/s00244-024-01067-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-024-01067-9