Abstract
The oceans become increasingly contaminated as a result of global industrial production and consumer behaviour, and this affects wildlife in areas far removed from sources of pollution. Migratory seabirds such as storm-petrels may forage in areas with different contaminant levels throughout the annual cycle and may show a carry-over of mercury from the winter quarters to the breeding sites. In this study, we compared mercury levels among seven species of storm-petrels breeding on the Antarctic South Shetlands and subantarctic Kerguelen Islands, in temperate waters of the Chatham Islands, New Zealand, and in temperate waters of the Pacific off Mexico. We tested for differences in the level of contamination associated with breeding and inter-breeding distribution and trophic position. We collected inert body feathers and metabolically active blood samples in ten colonies, reflecting long-term (feathers) and short-term (blood) exposures during different periods ranging from early non-breeding (moult) to late breeding. Feathers represent mercury accumulated over the annual cycle between two successive moults. Mercury concentrations in feathers ranged over more than an order of magnitude among species, being lowest in subantarctic Grey-backed Storm-petrels (0.5 μg g−1 dw) and highest in subtropical Leach’s Storm-petrels (7.6 μg g−1 dw, i.e. posing a moderate toxicological risk). Among Antarctic Storm-petrels, Black-bellied Storm-petrels had threefold higher values than Wilson’s Storm-petrels, and in both species, birds from the South Shetlands (Antarctica) had threefold higher values than birds from Kerguelen (subantarctic Indian Ocean). Blood represents mercury taken up over several weeks, and showed similar trends, being lowest in Grey-backed Storm-petrels from Kerguelen (0.5 μg g−1 dw) and highest in Leach’s Storm-petrels (3.6 μg g−1 dw). Among Antarctic storm-petrels, species differences in the blood samples were similar to those in feathers, but site differences were less consistent. Over the breeding season, mercury decreased in blood samples of Antarctic Wilson’s Storm-petrels, but did not change in Wilson’s Storm-petrels from Kerguelen or in Antarctic Black-bellied Storm-petrels. In summary, we found that mercury concentrations in storm-petrels varied due to the distribution of species and differences in prey choice. Depending on prey choices, Antarctic storm-petrels can have similar mercury concentrations as temperate species. The lowest contamination was observed in subantarctic species and populations. The study shows how seabirds, which accumulate dietary pollutants in their tissues in the breeding and non-breeding seasons, can be used to survey marine pollution. Storm-petrels with their wide distributions and relatively low trophic levels may be especially useful, but more detailed knowledge on their prey choice and distributions is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Marine ecosystems are major repositories of environmental contaminants. Seabirds have been widely used to monitor pollution of marine ecosystems, as they are long-lived and forage frequently at the apex of marine food webs (e.g. van Franeker and Bell 1988; Carravieri et al. 2014a, b, c). Using seabirds as bioindicators of marine contamination provides insight into the risk of human and other wildlife exposure to environmental contamination.
Mercury (Hg) is a toxic pervasive heavy metal occurring naturally in the environment. Nevertheless, anthropogenic activities, which currently represent two thirds of the global emissions (Pacyna et al. 2006) have substantially modified the cycling of this trace element on a global scale since pre-industrial times, mainly via fossil fuel combustion, industrial and agricultural residues, waste incineration and gold mining. Consequently, while Hg concentrations in the first one hundred metres in the ocean have doubled, they increased by 25% in deep waters (Lamborg et al. 2014). Hg can lead to deleterious effects on animals even at low doses, by affecting their nervous, reproductive and immune systems (Wolfe et al. 1998; Tan et al. 2009), with potential impacts at the population level (Goutte et al. 2014a, b).
High concentrations of Hg can be found not only in the vicinity of pollution sources, but also in remote environments. Because the elemental form of this metal is highly volatile and has a long atmospheric residence time (6 months to 1 year; Selin 2009), it is transported over long distances, reaching remote areas such as sub-polar and polar regions (Fitzgerald et al. 1998). When this element has been taken up from the atmosphere to the ocean, it is transformed into methyl mercury (MeHg) by microbial methylation in the water column and in sediments. Because of its high assimilation efficiency and high affinity for proteins, MeHg easily bioaccumulates in marine organisms (concentrations increase over time in their tissues) and biomagnifies in the food chains (concentrations increase at each trophic level) up to top predators resulting in elevated concentrations in seabirds. Only MeHg is biomagnified and not inorganic Hg. In seabird feathers and blood, Hg is mainly (> 90%) under the methylated form (see for instance Renedo et al. 2017, 2018), so THg is a proxy of MeHg in these tissues.
Elevated levels of MeHg contamination have been reported in seabirds from the Southern Ocean (Anderson et al. 2009; Carravieri et al. 2014a, b, c; Becker et al. 2016). In Arctic ecosystems, Dietz et al. (2009) determined that 92% of the Hg body burden accumulated in top predators result from anthropogenic activities.
As long-lived animals, seabirds accumulate dietary pollutants in their tissues in the breeding and non-breeding seasons. When the plumage is renewed, 70–90% of their accumulated Hg is excreted into their growing feathers (Agusa et al. 2005; Braune and Gaskin 1987; Honda et al. 1986). Thus, Hg concentrations in feathers reflect the year-round (long-term) Hg contamination of a bird (Thompson et al. 1998). In contrast to feathers which reflect the contamination since the last moult, blood reflects shorter-term (weeks up to 2 months) exposure (Albert et al. 2019), as well as any residual burden not yet depurated during feather moult (Bearhop et al. 2000). Thus, blood may be used to test for differences and carry-over of Hg among seasons. For example, Double-crested Cormorants (Phalacrocorax auritus) and Caspian Terns (Hydroprogne caspia) with winter sites with high Hg exposure still had elevated blood Hg values in summer (Lavoie et al. 2014).
Storm-petrels (families Oceanitidae and Hydrobatidae) are the smallest marine birds. They breed in cavities on remote, predator-free islands and most species migrate during the non-breeding season (Table 1). Their life cycle is characterized by annual breeding attempts with single-egg clutches, and a monogamous mating system (Quillfeldt et al. 2001) with intensive biparental care during incubation and chick-rearing.
In the present study, we focussed on storm-petrels from Antarctic and subantarctic breeding sites and also analysed samples from storm-petrels breeding in the North-East Pacific to enable a comparison with a less remote area. We tested for differences in the level of contamination associated with breeding and inter-breeding distribution and trophic position (determined using compound-specific stable isotope analyses). We further compared samples collected during the early and late Antarctic breeding season to quantify carry-over effects of the exposure to Hg in the inter-breeding season. Specifically, we wanted to test the hypotheses:
-
1.
The level of contamination increases with a higher trophic position (as a result of Hg biomagnification along food webs).
-
2.
The level of contamination increases with a more northerly breeding and inter-breeding distribution (lower contamination in Antarctic waters). For example, albatrosses feeding in Antarctic waters had lower Hg exposure compared to feeding at lower latitudes (Carravieri et al. 2014a; Cherel et al. 2018).
-
3.
The level of contamination is higher at the beginning of the season for Antarctic species (reflecting their higher contamination with Hg at lower latitudes during the non-breeding season). We expected that over the course of the Antarctic breeding season, the contamination levels drop as birds spend more time in Antarctic waters that are less contaminated. This has been shown in black-legged kittiwakes from Svalbard, which spent their breeding season in less contaminated waters (Tartu et al. 2022), as well as in Blue petrels (Quillfeldt et al. 2022a).
Materials and Methods
Study Sites and Species
Adult birds were generally captured by hand or mistnet in the colony when arriving from foraging after nightfall. All birds were released at the location of capture immediately after sampling. The present knowledge on the distribution and diet (Fig. 1) of the species (Winkler et al. 2020a, b) is summarized in Table 1.
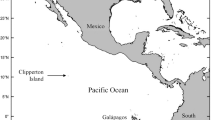
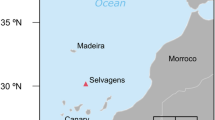
Map of the overall (multi-colony) year-round storm-petrel distributions of the seven species in this study. The central panel gives an overview of the distribution and shows the location of the study sites: Mex = Isla Coronado und Isla Todos Santos, Mexico, Ant = King George Island/25 de Mayo Island, South Shetland Islands, Antarctica, Ker = Mayes Island, Kerguelen Islands, NZ = Rangatira, Chatham Islands, New Zealand. Details are given in panel a–d: a Northern hemisphere species, b Northern hemisphere distribution of non-breeding Antarctic Storm-petrels. c + d Southern hemisphere species. Species: ASSP = Ashy Storm-petrel, BBSP = Black-bellied Storm-petrel, BLSP = Black Storm-petrel, GBSP = Grey-backed Storm-petrel, LESP = Leach’s Storm-petrel, WFSP = White-faced Storm-petrel, WISP = Wilson’s Storm-petrel, Distribution data and map source: https://mapper.obis.org
In the Antarctic, we sampled Wilson’s Storm-petrels (WISP) Oceanites oceanicus and Black-bellied Storm-petrels (BBSP) Fregetta tropica breeding at the Tres Hermanos (Three Brothers Hill) colony on King George Island/25 de Mayo Island, South Shetland Islands (62°14’S, 58°40’W). Birds were trapped using a 12 m mistnet between 20 November and 26 December 2017 (incubation), and 30 January and 28 February 2020 (chick-rearing). WISP and BBSP lay eggs from mid-December, and the peak of chick hatching is in the first half of February, but there is considerable variation in hatching date within the colony (e.g. 49 d time span in 1996; Quillfeldt and Peter 2000). Chicks are fed by their parents until fledging in the second half of March. The two sympatric species differ in their diet composition during the breeding season. While Black-bellied Storm-petrels take fish and crustaceans in equal proportions (Hahn 1998), Wilson’s Storm-petrels take mainly crustaceans (80–90% occurrence), feeding predominantly on Antarctic krill Euphausia superba (Quillfeldt 2002). Preliminary results from these two populations were presented in a conference (Quillfeldt et al. 2022b).
In the subantarctic, we sampled WISP and BBSP, as well as Grey-backed Storm-petrels (GBSP) Garrodia nereis breeding at Ile Mayes (49°28’S, 69°57’E) in the Kerguelen archipelago. Birds were trapped using mistnets between 5 and 12 December 2018 (incubation), and 22 February and 9 March 2019 (chick-rearing). In Crozet Islands in the Indian Ocean, WISP took mainly amphipods Themisto gaudichaudii and Euphausia vallentini, together with some copepods and cyprid larvae of cirripeds (Ridoux 1994). BBSP took the same crustaceans, but most of their prey was made up of fish and squid most likely obtained by scavenging (Ridoux 1994). GBSP have a diet specialized on Cirripedia (barnacle) larvae such as those of Lepas australis, a cold-water species inhabiting all the oceans surrounding the Antarctic continent (Newman an Ross 1971; Hinojosa et al. 2006). These pelagic barnacles attach to flotsam such as kelp and debris after spending up to 2 months drifting as larvae (Hinojosa et al. 2006). They are omnivores feeding on crustaceans and diatoms, and are among the higher trophic level animals in drifting seaweed communities (Sano et al. 2003).
In New Zealand, fieldwork was carried out in 2015 on South East Island (Hokorereoro/Rangatira: 44° 20′ S, 176° 10′ W). We collected samples from GBSP and White-faced Storm-petrels (WFSP) Pelagodroma marina from 25 November to 6 December 2015 (late incubation/hatching period). These data have previously been compared to other sympatric petrel species (Thébault et al. 2021).
In the Pacific Ocean, we sampled Black Storm-petrels (BLSP) Hydrobates melania, Leach’s Storm-petrels (LESP) Hydrobates leucorhous and Ashy Storm-petrels (ASSP) Hydrobates homochroa on Coronado Island (32°27′ N, 117°18′ W) from 11 to 15 July 2018 and additionally, Ashy Storm-petrels at Todos Santos Sur Island (31°48′ N, 116°47′ W) from 16 to 18 July 2018 (during the chick-rearing season).
Sample Preparation
Body feathers were plucked and conserved in sealed plastic bags until analysis. Body feathers are commonly considered as the best feather type to collect, since they are more representative of the entire plumage than other feather types and more homogenous (Furness et al. 1986). From a subsample of birds (N = 6 per colony), we analysed 4–12 individual feathers to compare the variability in stable isotope and Hg values within and among birds (361 feathers from 51 birds). Based on the results we obtained, the samples of the remaining birds were pooled for each individual for analysis.
Feathers were cleaned in a chloroform: methanol solution (2:1, v/v) in an ultrasonic bath and rinsed twice in methanol. After 48 h of drying at 45 °C in an oven, feathers were cut into tiny fragments with stainless steel scissors in order to obtain a homogenous powder.
Blood (0.2–0.4 ml) was sampled by puncturing the brachial wing vein and collected using heparinized capillaries, or using 0.3 ml syringes. Blood was stored and transported in ethanol. Storage in ethanol has been generally found to not affect the stable isotope values of blood samples (e.g. Hobson et al. 1997; Halley et al. 2008, but see Bugoni et al. 2008). In the laboratory blood samples were freeze-dried and ground to powder to be analysed for Hg and stable isotopes.
The half-life of isotope turnover for avian red blood cells in crows Corvus brachyrhynchos was 29.8 d (Hobson and Clark 1993) and 10.9 d for Yellow-rumped Warblers Dendroica coronate (Podlesak et al. 2005). Thus, blood samples collected from the small petrels in the present study likely represented the diet ingested ca. 2–4 weeks before sampling. Upon return to their breeding grounds, stable isotope values switch to values characteristic of their breeding season diet and location, suggesting minimal carry-over of isotopic signatures from diet and foraging areas during the non-breeding season (Lavoie et al. 2014).
However, significant carryover of Hg among seasons and slow changes in Hg over time have been observed, especially in individuals with high Hg exposure during non-breeding months (Lavoie et al. 2014; Quillfeldt et al. 2022a). This suggests that Hg is stored for long time periods in internal tissues and has a slow depuration rate. Hg is retained in the organism because renal excretion of MeHg is low and bile excretion is followed by intestinal reabsorption (Norseth and Clarkson 1971; Hirata and Takahashi 1981). A Hg carryover from wintering (non-breeding) sites indicates that Hg values measured in blood at a given time may be influenced by current uptake as well as the previous exposure from distant locations.
Mercury Analyses
Hg concentrations were determined as described in Bustamante et al. (2006). We measured aliquots (blood ~ 2 mg dry weight (dw), feathers ~ 1 mg dw) with an Advanced Mercury Analyser spectrophotometer Altec AMA-254. Measurements were repeated 2–3 × for each sample, until the relative standard deviation (RSD) was < 10%. For each measurement, accuracy and reproducibility were tested by including analytical blanks and certified reference materials (TORT-2: lobster hepatopancreas, certified concentration: 0.27 ± 0.06 μg g−1 dw; DOLT-5: dogfish liver, certified concentration: 0.44 ± 0.18 μg g−1 dw; National Research Council of Canada). Hg concentrations measured for the reference materials were: 0.26 ± 0.02 μg g−1 dw (n = 18) and 0.44 ± 0.01 μg g−1 dw (n = 7) for TORT-2 and DOLT-5 corresponding to a recovery rate of 96 ± 7% for TORT-2 and 100 ± 2% for DOLT-5. The limit of detection was 0.005 μg g−1 dw. Hg concentrations are expressed in μg g−1 dw.
Bulk Stable Isotope Analyses
In the Southern Ocean, δ13C values of seabirds correspond to the latitude of their foraging habitats (Quillfeldt et al. 2010; Jaeger et al. 2010), while δ15N values increase with trophic level (Cherel et al. 2010).
For bulk stable isotope analyses, an amount of 0.2–0.4 mg of subsample was weighed into tin cups. Carbon and nitrogen isotopic values were measured with a continuous-flow mass spectrometer (Thermo Scientific Delta V Advantage) coupled to an elemental analyser (Thermo Scientific Flash EA 1112). Internal laboratory standards (acetanilide and peptone) indicated a precision of ± 0.15‰ for both elements. Results are expressed in parts per thousand (‰) in the δ notation, relative to Vienna Pee Dee Belemnite for δ13C and atmospheric N2 for δ15N, following the formula:
where R is 13C/12C or 15N/14N, respectively.
In the Southern Ocean, we followed Cherel et al. (2018) to define distribution zones, based on feather δ13C isoscapes (Jaeger et al. 2010), as follows: Subtropical Zone (STZ): δ13C > − 18.3 ‰, Subantarctic Zone (SAZ): δ13C values of, − 21.2 to − 18.3 ‰, and Antarctic Zone (AZ): δ13C < − 21.2 ‰.
Compound-Specific Isotope Analyses of Amino Acids (CSIA-AA)
CSIA-AA can be used to provide a baseline-independent estimate of the trophic position of marine organisms from temporally and spatially variable environments. Therefore, it is especially suitable for this dataset spanning samples from different ocean basins and latitudes and thus, with different bulk stable isotope baselines.
CSIA-AA were performed at the UC Davis Stable Isotope facility (USA), as described previously (Quillfeldt & Masello 2020). Trophic positions (TP) were calculated from nitrogen stable isotope values of glutamic acid (Glx) and phenylalanine (Phe), using a stepwise trophic discrimination factor (multi-TDFGlx-Phe, with the equations:
For a detailed discussion, see Quillfeldt and Masello (2020). Small sample sizes were analysed with CSIA-AA due to the high costs (Tables 2 and 3).
Distribution Data
Distribution data were downloaded from GBIF.org on 08 October 2021, and plots for Fig. 1 from OBIS-SEAMAP (https://mapper.obis.org/, Halpin et al. 2009). GBIF data were summarized monthly (Fig. 2) and seasonally: the winter season was defined as June to August for southern hemisphere species, and December to February for northern hemisphere species. For WFSP, only southern hemisphere records were counted to exclude the northern hemisphere populations of this species.
Overall (multi-colony) year-round latitudinal distribution (mean and standard deviation) of storm-petrels in this study. Distribution data were downloaded from gbif.org. Species: ASSP = Ashy Storm-petrel, BBSP = Black-bellied Storm-petrel, BLSP = Black Storm-petrel, GBSP = Grey-backed Storm-petrel, LESP = Leach’s Storm-petrel, WFSP = White-faced Storm-petrel, WISP = Wilson’s Storm-petrel
Data Analyses
Data were analysed and visualized in R version 4.1.0. We used Shapiro tests and qq plots to test normality. Stable isotope and Hg values differed significantly from normal distribution. Therefore, univariate statistics on these were carried out with nonparametric tests, and the data were transformed using transform Tukey in the R package rcompanion before carrying out multivariate statistics such as linear models. A comparison of the model outputs did not show any major differences between models using transformed and untransformed data. To enhance readability, effect plots are thus given from models of untransformed data, i.e. showing the actual scale of the data. Means are given with standard deviations. To measure effect size, we included eta squared values (η2), obtained with the EtaSq function in the R package DescTools. To quantify intra-individual variabilities in stable isotope vales, we calculated repeatability values from six birds per species (but only two Black-bellied Storm-petrels from Kerguelen) following Lessels and Boag (1987).
To test nonlinear effects, Hg values were modelled using General additive models (GAMs) in the ‘mgcv’ package in R (Wood 2021). Cross-species models were developed separately for the northern and southern hemisphere populations, including species as random factor. In addition, separate models were developed for the populations. Models included δ13C and δ15N as fixed factors. The smoothing parameter was chosen automatically using generalized cross-validation. Contour plots were generated with the vis.gam function.
Results
Distribution
The distribution data of the three northern hemisphere species from sightings corresponding to multiple colonies (Fig. 2, upper panel) showed that ASSP remain close to their breeding sites in the winter (November–March), while BLSP move to more southern (i.e. tropical) waters. The mean distribution of Black Storm-petrels was in the tropics (< 23.3°S) between November and March. Leach’s Storm-petrels LESP had a wide distribution that overlapped with the latitude of their breeding distribution throughout the year. Overall, LESP tended to be more northerly in early winter (November–January) and in more southern latitudes in late winter (February–March).
Among the southern hemisphere species (Fig. 2, lower panel), GBSP remained close to the latitude of their breeding colony in the winter (April to October). WISP migrated to the northern hemisphere (mean lat. 37 °N) and BBSP and WFSP moved to subtropical and tropical waters. The mean distribution of BBSP was in the tropics (≥ − 23.3°S) in August and September.
Feather Samples
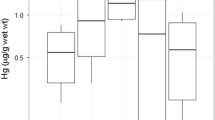
Low Hg concentrations < 5 μg g−1 were found in feathers of southern hemisphere species (Table 2, Fig. 3), with the exception of BBSP from King George Island/25 de Mayo Island. The Hg concentrations of those storm-petrels were close to those of the three northern hemisphere species, with elevated Hg concentrations > 5 μg g−1 (Table 2, Fig. 3). Among Antarctic storm-petrels, BBSP had threefold higher values than WISP. In both species, birds from the South Shetlands (Antarctica) had threefold higher values than birds from Kerguelen (Subantarctic Indian Ocean; t-test for BBSP: t = 5.3, df = 2.1, P = 0.032, t-test for WISP: t = 11.2, df = 65.4, P < 0.001).
Hg concentrations in feathers and blood samples of storm-petrels from ten populations. Species: ASSP = Ashy Storm-petrel, BBSP = Black-bellied Storm-petrel, BLSP = Black Storm-petrel, GBSP = Grey-backed Storm-petrel, LESP = Leach’s Storm-petrel, WFSP = White-faced Storm-petrel, WISP = Wilson’s Storm-petrel, Sites: KGI = King George Island/25 de Mayo Island (South Shetlands, Antarctic), KER = Kerguelen Islands (Southern Indian Ocean), MEX = Mexican Pacific Islands, CHA = Chatham Islands, New Zealand
The carbon stable isotope values of feathers showed a relatively narrow range (SD within − 19.5 to − 16.0 ‰, Fig. 4), indicating a wintering range from subtropical to temperate waters. Nitrogen stable isotope values had a larger range (9–19 ‰, Fig. 4). In a linear model across species, carbon stable isotope values did not correlate with Hg concentrations (F = 0.2, P = 0.653), but nitrogen stable isotope values were positively related to Hg concentrations (F = 7.9, P = 0.026, Fig. 4). Similarly, in models run across species, the trophic position according to CSIA-AA had a positive correlation with feather Hg values, while carbon stable isotope values did not correlate with Hg concentrations (Fig. S1). Overall, however, the strongest effects were those of species and site differences (Fig. S1).
Hg concentrations and stable isotope values in feathers of storm-petrels from ten populations. Species: ASSP = Ashy Storm-petrel, BBSP = Black-bellied Storm-petrel, BLSP = Black Storm-petrel, GBSP = Grey-backed Storm-petrel, LESP = leach’s Storm-petrel, WFSP = White-faced Storm-petrel, WISP = Wilson’s Storm-petrel
The trophic position according to CSIA-AA measured in feathers ranged from 2.9 to 4.0 (Table 2, Fig. S2), while the ranges for the species overlapped, a 0.5 TP difference was seen within the Antarctic storm-petrels, with higher trophic levels in BBSP than WISP (Fig. S2).
Feathers analysed individually for repeatability of Hg concentrations (N = 6 individuals per colony, 4–12 individual feathers per bird) showed different degrees of repeatability, from r < 0 (MEX_BLSP), over poor repeatability < 50% (MEX_LESP: 18%, KER_BBSP: 40%, KER_GBSP: 48%, KER_WISP: 48%), to moderate repeatability > 50% (KGI_WISP: 52%, MEX_ASSP: 58%). Only BBSP from King George Island/25 de Mayo Island (Antarctica) reached good repeatability > 80% (KGI_BBSP: 81%, Fig. S3).
Blood Samples
Species differences in Hg in blood samples mirrored those in feather samples, but at a narrower range (Fig. 3). Low Hg concentrations < 1.5 μg g−1 dw were found in feathers of the southern hemisphere species (Table 3, Fig. 3), with the exception of the BBSP. The Hg concentrations of those BBSP were close to those of the three northern hemisphere species, with elevated Hg concentrations > 5 μg g−1 (Table 2, Fig. 3). Among Antarctic storm-petrels, BBSP had threefold higher values than WISP. In contrast to Hg concentrations in feathers, birds from the South Shetlands (Antarctica) had similar blood Hg values to birds from Kerguelen (Subantarctic Indian Ocean; t-test for BBPS: t = 1.7, df = 1.3, P = 0.308, t-test for WISP: t = 0.8, df = 62.5, P = 0.385).
In Antarctic storm-petrels, Hg concentrations in blood consistently increased with increasing δ15N values (Figs. 5 and S4). We used GAMs to test for effects of foraging habitat (δ13C), trophic position (δ15N) and breeding stage (incubation vs. chick-rearing) on Hg concentrations in blood of Antarctic storm-petrels. We found increasing blood Hg with higher δ15N (Figs. S4 and 6, Table 4), while the influence of δ13C was both weaker (lower effect sizes), and less consistent in direction (Fig. S4). The breeding stage played a minor role, although Hg concentrations decreased in WISP on King George Island/25 de Mayo Island over the course of the breeding season (Fig. S4).
Temporal changes in Hg concentrations in blood of Wilson’s and Black-bellied Storm-petrels over the breeding season, in relation to stable isotope values. The size of the points is relative to the Hg concentration, and Wilcoxon tests correspond to comparisons of Hg concentrations between incubating and chick-rearing birds. Species: BBSP = Black-bellied Storm-petrel, WISP = Wilson’s Storm-petrel, Sites: KGI = King George Island/25 de Mayo Island (South Shetlands, Antarctic), KER = Kerguelen Islands (Southern Indian Ocean)
General additive model (GAM) fits of Hg values in feathers of storm-petrels breeding on King George Island/25 de Mayo Island (South Shetland, Antarctic) and Kerguelen Islands, in relation to carbon and nitrogen stable isotope values and the time in the breeding season. Species: BBSP = Black-bellied Storm-petrel, WISP = Wilson’s Storm-petrel, Sites: KGI = King George Island/25 de Mayo Island (South Shetlands, Antarctic), KER = Kerguelen (Southern Indian Ocean)
Similarly, in models run across species, the trophic position according to CSIA-AA had a positive correlation with blood Hg values, while carbon stable isotope values did not correlate with Hg concentrations (Fig. S1). Overall, however, the strongest effects were those of species and site differences (Fig. S1).
The trophic position according to CSIA-AA measured in blood ranged from 2.9 to 4.0 (Table 3, Fig. S2), while the ranges for species overlapped, a 0.3–0.4 TP difference was seen within the Antarctic storm-petrels, with higher trophic levels in BBSP than WISP (Table 3, Fig. S2).
Discussion
This study provides for the first time a large-scale assessment of Hg contamination in storm-petrels. While some populations (GBPS; WISP, BBSP from Kerguelen) showed values that are below toxicological risk thresholds, the BBSP from King George Island as well as the three northern hemisphere species (ASSP, BLSP, LESP) had feather Hg values above 5 µg/g dry weight, indicating a moderate toxicological risk (Burger and Gochfeld 1997).
Spatial Differences in Hg
We first tested whether the level of contamination increases with a species’ more northerly breeding and inter-breeding distribution as Antarctic waters seem to be less contaminated than more northern waters (evidence from several seabird species: Cherel et al. 2018; Carravieri et al. 2016, 2017, 2020). This hypothesis was largely supported, but a notable exception was BBSP that had higher feather and blood Hg concentrations than other Antarctic species. Their Hg levels were comparable to those of storm-petrels breeding in the Mexican Pacific Islands. To the best of our knowledge, few storm-petrel populations have been studied for Hg so far. In the northern hemisphere, studies focussed only on the Atlantic sector (Table S1). The present data from three storm-petrel species from the Eastern Pacific Ocean show a comparable level of Hg contamination to species in the Northern Atlantic (Pollet et al. 2022, Table S1a).
In feathers, elevated Hg concentrations were only observed in the BBSP population from King George Island/25 de Mayo Island (Antarctica), suggesting that they accumulate Hg during their yearly cycle, which includes winter months in tropical waters of the Atlantic or Pacific Ocean. In comparison, BBSP from the Kerguelen Islands had lower Hg values in feathers, suggesting that they had not accumulated such high Hg stores throughout their annual migration, which is probably restricted to the Indian Ocean. A comparison of Hg concentrations of individual feathers (subsamples of N = 6 per colony) suggested that feathers sampled from individual BBSP were relatively similar to each other (repeatability 81%) in the King George Island/25 de Mayo Island population.
In contrast, we observed high intra-individual variability in most of the storm-petrels studied here. This may be an indication of moult strategies and yearly movements. Storm-petrels have different moult strategies in relation to their breeding and wintering sites. As soon as Antarctic species such as the WISP finish breeding, they fly to their wintering areas where they moult rapidly (Scott 1970). In contrast, temperate species may begin moult late during nesting and require the entire non-breeding period to complete their moult (Scott 1970). Thus, in those temperate species higher variability in Hg in their plumage is expected. In addition, differences in their year-round ecology and movements may also determine the moult schedule. For example, Ainley et al. (1976) compared the moult of ASSP and LESP at South Farallon, California. ASSP is a short-ranging, sedentary species, while LESP is a long-ranging, migratory species. The moult began for both species with a renewal of body feathers. LESP started body moult in the fall and finished in the spring, taking a mean of 274 days. ASSP needed less time (257 days), and had a different timing, starting moult at the time their eggs had hatched, and showing peak scores in August when adults were feeding their chicks (Ainley et al. 1976). In our analysis, the highest repeatability values of intra-individual feather Hg concentrations were found in the two migratory Antarctic populations (WISP: 52%, BBSP: 81%), and the sedentary population (ASSP: 58%), while lowest repeatability values were found in migratory northern hemisphere populations (BLSP 0%, LESP: 18%). This suggests that the latter two populations have extended moult durations over which they use waters of different Hg contamination degree or different prey, including breeding site, migration and winter areas. In albatross, prolonged moulting has been shown to affect the feather Hg concentrations dramatically (Cherel et al. 2018). In contrast, Antarctic storm-petrels moult after arrival to their winter site and at a faster rate (e.g. 4 months or 120 days in WISP, Stresemann and Stresemann 1966), leading to less intra-individual variability.
Species Differences and Trophic Position
We further aimed to test if the level of contamination increases with the trophic position. We found some support for this hypothesis, but also some discordance in the results.
Among species, a linear model for feathers and blood supported a positive correlation between the trophic position according to CSIA-AA and Hg concentrations (Fig. S1). However, the species and site effects were much more important than those of the trophic position. This may be explained by the relatively similar trophic positions among storm-petrels, but also by the differences in time integration: while stable isotopes in feathers integrate nutrients taken up during the moulting time, Hg concentrations reflect the cumulative annual burden.
In northern hemisphere habitats such as the California current, higher baseline δ15N values (e.g. Bedolla-Guzmán et al. 2021) were observed in seabirds, in line with our measurements (e.g. Figure 7). In a study comparing BLSP and LESP using stable isotopes, both species preyed on fish larvae in similar proportions (about 50%), but BLSP consumed higher trophic level krill (including Nyctiphanes simplex, Nematoscelis difficilis, and Thysanoessa spinifera), resulting in higher δ15N values (e.g. Bedolla-Guzmán et al. 2021). In our study, ASSP had the highest δ15N values in feathers and blood of the three species in the Mexican Pacific, but had similar Hg values to the other two species. These observations suggest that although the trophic position is an important factor influencing Hg contamination, other species-specific factors may also play a role, such as the type of prey. For example, myctophids can play an important role in the diet of storm-petrels (e.g. Croxall and North 1988; Vermeer and Devito 1988). Thus, all otoliths found in BBSP at King George Island/25 de Mayo Island were from the myctophid Electrona antarctica (Hahn 1998), and myctophids were key components of LESP in Atlantic Canada (Frith et al. 2020). Myctophids have notably high Hg concentrations (e.g. Seco et al. 2020: myctophid Electrona antarctica: 0.18 ± 0.09 μg g−1) compared to krill (Seco et al. 2019: Euphausia tricantha: 0.03 ± 0.01 μg g−1) and other higher trophic level prey such as predatory amphipods (Seco et al. 2021: Themisto gaudichaudii: 0.04 ± 0.01 μg g−1). Similar elevated Hg concentrations in myctophids compared to zooplankton species were reported in subantarctic Kerguelen waters (Bustamante et al. 2003; Cipro et al. 2018). Myctophids are mesopelagic and spend the day at the oxygen minimum zone where anaerobic microorganisms methylate inorganic Hg into MeHg (Martin et al. 2006). Consequently, storm-petrels that feed on myctophid fish coming to the surface at night may have high Hg concentrations in their tissues (Elliott and Elliott 2016). Feeding on mesopelagic prey explains high Hg concentrations in seabirds (Ochoa-Acuña et al. 2002) and thus, high Hg concentrations in BBSP from this study might result from feeding on myctophids, and species differences among the sympatric species in the Mexican Pacific.
Hg concentrations and stable isotope values in blood of storm-petrels from ten populations, sampled during the chick-provisioning period. Species: ASSP = Ashy Storm-petrel, BBSP = Black-bellied Storm-petrel, BLSP = Black Storm-petrel, GBSP = Grey-backed Storm-petrel, LESP = Leach’s Storm-petrel, WFSP = White-faced Storm-petrel, WISP = Wilson’s Storm-petrel
Seasonal Differences in Hg
By comparing blood samples collected early and late in the breeding season of the two Antarctic storm-petrel species, we aimed to test the hypothesis that the level of contamination is higher at the beginning of the season, as a result of carry-over from the winter areas. Over the course of the season, the contamination level should drop as birds spend more time in Antarctic waters where they feed on prey with lower Hg concentrations compare to the prey from lower latitudes. Hg decreases in blood over the course of the breeding season have been shown in seabirds which migrate to more contaminated areas in the non-breeding season (e.g. Double-Crested Cormorants Phalacrocorax auratus: Lavoie et al. 2014, Blue Petrels Halobaena caerulea: Quillfeldt et al. 2022a, Black-legged Kittiwakes: Tartu et al. 2022; Great skua Stercorarius skua: Albert et al. 2022). In the present study, a decrease was seen for WISP on King George Island/25 de Mayo Island (Antarctica), but not for WISP on the Kerguelen Islands (Southern Indian Ocean) or BBSP on King George Island/25 de Mayo Island. This suggests that for these latter populations, Hg exposure is similar in the breeding and non-breeding season. Colony-specific distributions from tracking data would be needed to explain these patterns.
Conclusion
In summary, Hg contamination varied considerably among storm-petrels with large differences among species and sites. Even Antarctic storm-petrels that are far removed from sources of pollution, can experience considerable Hg contamination. The lowest contamination was observed in subantarctic species and populations. Further research is needed on the distribution and diet of these species, as well as the Hg contamination of their prey, in order to fully understand the observed patterns.
Availability of data and materials
All raw data will be submitted to the PANGAEA database. Until publication in the database, raw data are available from the authors.
References
Agusa T, Matsumoto T, Ikemoto T, Anan Y, Kubota R, Yasunaga G, Kunito T, Tanabe S, Ogi H, Shibata Y (2005) Body distribution of trace elements in Blacktailed gulls from Rishiri Island, Japan: age-dependent accumulation and transfer to feathers and eggs. Environ Toxicol Chem 24:2107
Ainley DG, Lewis TJ, Morrell S (1976) Molt in Leach’s and Ashy Storm-Petrels. The Wilson Bulletin 88:76–95
Albert C, Renedo M, Bustamante P, Fort J (2019) Using blood and feathers to investigate large-scale Hg contamination in Arctic seabirds: a review. Environ Res 177:108588
Albert C, Strøm H, Helgason HH, Bråthen VS, Gudmundsson FT, Bustamante P, Fort J (2022) Spatial variations in winter Hg contamination affect egg volume in an Arctic seabird, the great skua (Stercorarius skua). Environ Pollut 314:120322
Anderson ORJ, Phillips RA, McDonald RA, Shore RF, McGill RAR, Bearhop S (2009) Influence of trophic position and foraging range on mercury levels within a seabird community. Mar Ecol Prog Ser 375:277–288
Bearhop S, Ruxton GD, Furness RW (2000) Dynamics of mercury in blood and feathers of great skuas. Environ Toxicol Chem 19:1638–1643
Becker PH, Goutner V, Ryan PG, González-Solís J (2016) Feather mercury concentrations in Southern Ocean seabirds: variation by species, site and time. Environ Pollut 216:253–263
Bedolla-Guzmán Y, Masello JF, Aguirre-Muñoz A, Lavaniegos BE, Voigt CC, Gómez-Gutiérrez J, Sánchez-Velasco L, Robinson CJ, Quillfeldt P (2021) Year-round niche segregation of three sympatric Hydrobates Storm-petrels from Baja California Peninsula, Mexico, Eastern Pacific. Mar Ecol Prog Ser 664:207–225
Braune BM, Gaskin DE (1987) Hg levels in Bonaparte’s Gulls (Larus philadelphia) during autumn molt in the Quoddy Region, New Brunswick, Canada. Arch Environ Contam Toxicol 16:539–549
Bugoni L, McGill RAR, Furness RW (2008) Effects of preservation methods on stable isotopes signatures in bird tissues. Rapid Commun Mass Spectrom 22:2457–2462
Burger J, Gochfeld M (1997) Risk, mercury levels, and birds: relating adverse laboratory effects to field biomonitoring. Environ Res 75:160–172
Bustamante P, Bocher P, Cherel Y, Miramand P, Caurant F (2003) Distribution of trace elements in the tissues of benthic and pelagic fish from the Kerguelen Islands. Sci Total Environ 313:25–39
Bustamante P, Lahaye V, Durnez C, Churlaud C, Caurant F (2006) Total and organic Hg concentrations in cephalopods from the North East Atlantic waters: influence of geographical origin and feeding ecology. Sci Total Environ 368:585–596
Carravieri A, Bustamante P, Tartu S, Meillère A, Labadie P, Budzinski H, Peluhet L, Barbraud C, Weimerskirch H, Chastel O, Cherel Y (2014a) Wandering albatrosses document latitudinal variations in the transfer of persistent organic pollutants and mercury to Southern Ocean predators. Environ Sci Technol 48:14746–14755
Carravieri A, Cherel Y, Blévin P, Brault-Favrou M, Chastel O, Bustamante P (2014b) Mercury exposure in a large subantarctic avian community. Environ Pollut 190:51–57
Carravieri A, Bustamante P, Churlaud C, Fromant A, Cherel Y (2014c) Moulting patterns drive within-individual variations of stable isotopes and mercury in seabird body feathers: Implications for monitoring of the marine environment. Mar Biol 161:963–968
Carravieri A, Cherel Y, Jaeger A, Churlaud C, Bustamante P (2016) Penguins as bioindicators of mercury contamination in the southern Indian Ocean: geographical and temporal trends. Environ Pollut 213:195–205. https://doi.org/10.1016/j.envpol.2016.02.010
Carravieri A, Cherel Y, Brault-Favrou M, Churlaud C, Peluhet L, Labadie P, Budzinski H, Chastel O, Bustamante P (2017) From Antarctica to the subtropics: contrasted geographical concentrations of selenium, mercury, and persistent organic pollutants in skua chicks (Catharacta spp.). Environ Pollut 228:464–473
Carravieri A, Bustamante P, Labadie P, Budzinski H, Chastel O, Cherel Y (2020) Trace elements and persistent organic pollutants in chicks of 13 seabird species from Antarctica to the subtropics. Environ Int 134:105225
Cherel Y, Fontaine C, Richard P, Labat JP (2010) Isotopic niches and trophic levels of myctophid fishes and their predators in the Southern Ocean. Limnol Oceanogr 55:324–332
Cherel Y, Barbraud C, Lahournat M, Jaeger A, Jaquemet S, Wanless RM, Phillips RA, Thompson DR, Bustamante P (2018) Accumulate or eliminate? Seasonal mercury dynamics in albatrosses, the most contaminated family of birds. Environ Pollut 241:124–135
Cipro CV, Cherel Y, Bocher P, Caurant F, Miramand P, Bustamante P (2018) Trace elements in invertebrates and fish from Kerguelen waters, southern Indian Ocean. Polar Biol 41:175–191
Croxall JP, North AW (1988) Fish prey of Wilson’s storm petrel Oceanites oceanicus at South Georgia. British Antarctic Survey Bulletin 78:37–42
Dietz R, Outridge PM, Hobson KA (2009) Anthropogenic contributions to mercury levels in present-day Arctic animals—a review. Sci Total Environ 407(24):6120–6131
Elliott KH, Elliott JE (2016) Origin of sulfur in diet drives spatial and temporal mercury trends in seabird eggs from Pacific Canada 1968–2015. Environ Sci Technol 50:13380–13386
Fitzgerald WF, Engstrom DR, Mason RP, Nater EA (1998) The case for atmospheric mercury contamination in remote areas. Environ Sci Technol 32:1–7
Frith R, Krug D, Ronconi RA, Wong SN, Mallory ML, Tranquilla LA (2020) Diet of Leach’s storm-petrels (Hydrobates leucorhous) among three colonies in Atlantic Canada. Northeast Nat 27:612–630
Furness RW, Muirhead SJ, Woodburn M (1986) Using bird feathers to measure mercury in the environment: Relationships between mercury content and moult. Mar Pollut Bull 17:27–30
Goutte A, Barbraud C, Meillère A, Carravieri A, Bustamante P, Labadie P, Budzinski H, Delord K, Cherel Y, Weimeskirch H, Chastel O (2014a) Demographic consequences of heavy metals and persistent organic pollutants in a vulnerable long-lived bird, the wandering albatross. Proc R Soc B 281:20133313
Goutte A, Bustamante P, Barbraud C, Delord K, Weimeskirch H, Chastel O (2014b) Demographic responses to mercury exposure in two closely-related Antarctic top predators. Ecology 95(4):1075–1086
Hahn S (1998) The food and chick feeding of black-bellied Storm-petrel (Fregetta tropica) at King George Island, South Shetlands. Polar Biol 19:354–357
Halley DJ, Minagawa M, Nieminen M, Gaare E (2008) Preservation in 70 % ethanol solution does not affect δ13C and δ15N values of reindeer blood samples—relevance for stable isotope studies of diet. Rangifer 28:9–12
Halpin PN, Read AJ, Fujioka E, Best BD, Donnelly B, Hazen LJ, Kot C, Urian K, LaBrecque E, Dimatteo A, Cleary J, Good C, Crowder LB, Hyrenbach KD (2009) OBIS-SEAMAP: The world data center for marine mammal, sea bird, and sea turtle distributions. Oceanography 22(2):104–115
Hinojosa I, Boltana S, Lancellotti D, Macaya E, Ugalde P, Valdivia N, Vasquez N, Newman W, Thiel M (2006) Geographic distribution and description of four pelagic barnacles along the south east Pacific coast of Chile–a zoogeographical approximation. Rev Chil Hist Nat 79:13–27
Hirata E, Takahashi H (1981) Degradation of methyl mercury glutathione by the pancreatic enzymes in bile. Toxicol Appl Pharmacol 58(3):483–491
Hobson KA, Clark RG (1993) Turnover of 13C cellular and plasma reactions of blood: implications for non-destructive sampling in avian dietary studies. Auk 110:638–641
Hobson KA, Gibbs HL, Gloutney ML (1997) Preservation of blood and tissue samples for stable-carbon and stable nitrogen isotope analysis. Can J Zool 75:1720–1723
Honda K, Nasu T, Tatsukawa R (1986) Seasonal changes in Hg accumulation in the black-eared kite, Milvus migrans lineatus. Environ Pollut Ecol Biol 42:325–334
Jaeger A, Lecomte VJ, Weimerskirch H, Richard P, Cherel Y (2010) Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators’ foraging areas in the Southern Ocean. Rapid Commun Mass Spectrometry 24:3456–3460
Lamborg CH, Hammerschmidt CR, Bowman KL, Swarr GJ, Munson KM, Ohnemus DC, Lam PJ, Heimbürger LE, Rijkenberg MJA, Saito MA (2014) A global ocean inventory of anthropogenic mercury based on water column measurements. Nature 512:65–68
Lavoie RA, Baird CJ, King LE, Kyser TK, Friesen VL, Campbell LM (2014) Contamination of mercury during the wintering period influences concentrations at breeding sites in two migratory piscivorous birds. Environ Sci Technol 48:13694–13702
Lessels CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Martin I, Costa V, Porteiro FM, Santos RS (2006) Temporal and spatial changes in mercury concentrations in the North Atlantic as indicated by museum specimens of glacier lanternfish Benthosema glaciale (Pisces: Myctophidae). Environ Toxicol 21:528–532
Newman WA, Ross A (1971) Antarctic cirripedia. Antarctic Research Series 14. Washington DC: American Geophysical Union.
Norseth T, Clarkson TW (1971) Intestinal transport of 203Hg-labeled methyl mercury chloride. Arch Environ Health Int J 22(5):568–577
Ochoa-Acuña H, Sepúlveda MS, Gross TS (2002) Mercury in feathers from Chilean birds: influence of location, feeding strategy, and taxonomic affiliation. Mar Pollut Bull 44:340–345
Pacyna EG, Pacyna JM, Steenhuisen F, Wilson S (2006) Global anthropogenic mercury emission inventory for 2000. Atmos Environ 40:4048–4063
Podlesak DW, McWilliams SR, Hatch KA (2005) Stable isotopes in breath, blood, feces and feathers can indicate intra-individual changes in the diet of migratory songbirds. Oecologia 142:501–510
Pollet IL, Provencher JF, Tranquilla LM, Burgess NM, Mallory ML (2022) Mercury levels in North Atlantic seabirds: a synthesis. Mar Pollut Bull 181:113884
Quillfeldt P (2002) Seasonal and annual variation in the diet of breeding and non-breeding Wilson’s Storm-petrels on King George Island, South Shetland Islands. Polar Biol 25:216–221
Quillfeldt P, Masello JF (2020) Compound-specific stable isotope analyses in Falkland Islands seabirds reveal seasonal changes in trophic positions. BMC Ecol 20:1–12
Quillfeldt P, Peter HU (2000) Provisioning and growth in chicks of Wilson’s Storm-petrels Oceanites oceanicus on King George Island, South Shetland Islands. Polar Biol 23:817–824
Quillfeldt P, Masello JF, McGill RA, Adams M, Furness RW (2010) Moving polewards in winter: a recent change in the migratory strategy of a pelagic seabird? Front Zool 7:15
Quillfeldt P, Cherel Y, Navarro J, Phillips RA, Masello JF, Suazo CG, Delord K, Bustamante P (2022a) Variation among species and populations, and carry-over effects of winter exposure on mercury accumulation in small petrels. Front Ecol Evolut 10: Article 915199.
Quillfeldt P, Kraemer P, Thébault J, Libertelli MM, Bustamante P (2022b) Poster abstract– DFG SPP 1158 Antarctic Research—Report Colloquium. Distribution and mercury contamination in Antarctic storm petrels. Reports on Polar and Marine Research 762:119.
Renedo M, Bustamante P, Tessier E, Pedrero Z, Cherel Y, Amouroux D (2017) Assessment of mercury speciation in feathers using species-specific isotope dilution analysis. Talanta 174:100–110
Renedo M, Amouroux D, Duval B, Carravieri A, Tessier E, Barre J, Bérail S, Pedrero Z, Cherel Y, Bustamante P (2018) Seabird tissues as efficient biomonitoring tools for Hg isotopic investigations: implications of using blood and feathers from chicks and adults. Environ Sci Technol 52:4227–4234
Ridoux V (1994) The diet and dietary segregation of seabirds at the subantarctic Crozet islands. Mar Ornithol 22:1–192
Sano M, Michio O, Kazuya T (2003) Predator-prey systems of drifting seaweed communities off the Tohoku coast, northern Japan, as determined by feeding habit analysis of phytal animals. Fish Sci 69:260–268
Scott DA (1970) The breeding biology of the Storm Petrel Hydrobates pelagicus. Unpubl. doctoral dissertation, University of Oxford
Seco J, Xavier JC, Coelho JP, Pereira B, Tarling G, Pardal MA, Pereira ME (2019) Spatial variability in total and organic mercury levels in Antarctic krill Euphausia superba across the Scotia Sea. Environ Pollut 247:332–339
Seco, J., Xavier, J. C., Bustamante, P., Coelho, J. P., Saunders, R. A., Ferreira, N, Fielding S, Parda MA, Stowasser G, Viana T, Tarling GA, Pereira E, Brierley AS (2020) Main drivers of mercury levels in Southern Ocean lantern fish Myctophidae. Environ Pollut 264:114711.
Seco J, Aparício S, Brierley AS, Bustamante P, Ceia FR, Coelho J, Philips RA, Saunders RA, Fielding S, Gregory S, Matias R (2021) Mercury biomagnification in a Southern Ocean food web. Environ Pollut 275:116620
Selin NE (2009) Global biogeochemical cycling of mercury: a review. Annu Rev Environ Resour 34:43–63
Stresemann E, Stresemann V (1966) Die Mauser der Vögel. J Ornithol 107:1–448
Tan SW, Meiller JC, Mahaffey KR (2009) The endocrine effects of mercury in humans and wildlife. Crit Rev Toxicol 39:228–269
Tartu S, Blévin P, Bustamante P, Angelier F, Bech C, Bustnes JO, Chierici M, Fransson A, Gabrielsen GW, Goutte A, Moe B (2022) A U-turn for mercury concentrations over 20 years: How do environmental conditions affect exposure in Arctic seabirds? Environ Sci Technol 56:2443–2454
Thébault J, Bustamante P, Massaro M, Taylor G, Quillfeldt P (2021) Influence of species-specific feeding ecology on mercury concentrations in seabirds breeding on the Chatham Islands, New Zealand. Environ Toxicol Chem 40:454–472
Thompson DR, Bearhop S, Speakman JR, Furness RW (1998) Feathers as a means of monitoring mercury in seabirds: insights from stable isotope analysis. Environ Pollut 101(2):193–200
van Franeker JA, Bell PJ (1988) Plastic ingestion by petrels breeding in Antarctica. Mar Pollut Bull 19:672–674
Vermeer K, Devito K (1988) The importance of Paracallisoma coecus and myctophid fishes to nesting fork-tailed and Leach’s storm-petrels in the Queen Charlotte Islands, British Columbia. J Plankton Res 10:63–75
Winkler DW, Billerman SM, Lovette IJ (2020a). Southern Storm-Petrels (Oceanitidae), version 1.0. In Birds of the World (S. M. Billerman, B. K. Keeney, P. G. Rodewald, and T. S. Schulenberg, Editors). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.oceani2.01. Accessed on 15 November 2021
Winkler DW, Billerman SM, Lovette IJ (2020b) Northern Storm-Petrels (Hydrobatidae), version 1.0. In Birds of the World. In: Billerman SM, Keeney BK, Rodewald, PG Schulenberg TS (eds) Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.hydrob1.01. Accessed on 15 November 2021
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17:146–160
Wood S (2021) Mixed GAM Computation Vehicle with Automatic Smoothness Estimation. https://cran.r-project.org/web/packages/mgcv/mgcv.pdf. Accessed on 26 November 2021
Acknowledgements
We thank Justine Thébault for collecting samples in the field and preparing samples for analyses, and Yvonne Schumm, Phillip Krämer and Miguel Corrales Sauceda for help with fieldwork. We appreciate the support of C. Churlaud and M. Brault-Favrou from the Plateforme Analyses Elémentaires of the LIENSs laboratory during Hg analyses, and of G. Guillou from the Plateforme Analyses Isotopiques of the LIENSs laboratory for running stable isotope analyses. We gratefully acknowledge helpful comments from two anonymous reviewers. A poster with some preliminary results (restricted to Wilson’s Storm-petrel and Black-bellied Storm-petrels) was presented at the meeting of the German Society for Polar Research in Potsdam, 01–05 May 2022 (https://epic.awi.de/id/eprint/55943/1/BzPM_0762_2022.pdf).
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the Deutsche Forschungsgemeinschaft (DFG) in the framework of the priority programme SPP1154 “Antarctic Research with comparative investigations in Arctic ice areas” (grant Qu148/18). The Argentinean Antarctic Institute provided financial and logistical support to carry out species sampling in Antarctica. Thanks to the CPER (Contrat de Projet Etat-Région) and the FEDER (Fonds Européen de Développement Régional) for funding the AMA, and the IRMS of LIENSs laboratory. Thanks to the David and Lucile Packard Foundation and the Marisla Foundation for funding fieldwork in Mexico. The IUF (Institut Universitaire de France) is acknowledged for its support to P. Bustamante as a Senior Member.
Author information
Authors and Affiliations
Contributions
PQ and PB conceived and designed the study. PQ, YB, YC and MM were involved in organizing and carrying out the fieldwork, and JT and PB carried out the laboratory work. PQ carried out the data analyses. PQ and PB drafted the manuscript. All authors reviewed the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study involved wild individuals and was carried out under permits from the Argentine Antarctic Institute IAA, the Animal Ethics Committee of the Institut Polaire Français Paul Emile Victor (Programme N°109, C. Barbraud), Préfet des Terres Australes et Antarctiques Françaises, the Animal Ethics Committee of Charles Sturt University and the Department of Conservation, New Zealand, and Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT) and Secretaría de Gobernación (SEGOB), Mexico.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quillfeldt, P., Bedolla-Guzmán, Y., Libertelli, M.M. et al. Mercury in Ten Storm-Petrel Populations from the Antarctic to the Subtropics. Arch Environ Contam Toxicol 85, 55–72 (2023). https://doi.org/10.1007/s00244-023-01011-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-023-01011-3