Abstract
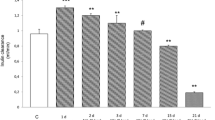
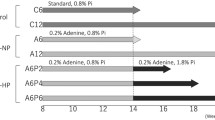
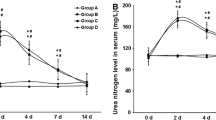
Metabolic syndrome (MS) individuals have a higher risk of developing chronic kidney disease through unclear pathogenic mechanisms. MS has been also related with higher nephrolithiasis prevalence. To establish the influence of MS on renal function, we designed a murine model of combined metabolic syndrome and hyperoxaluria. Four groups of male Sprague–Dawley rats were established: (1) control group (n = 10) fed with standard chow; (2) stone former group (SF) (n = 10) fed with standard chow plus 0.75% ethylene glycol administered in the drinking water; (3) metabolic syndrome group (MS) (n = 10), fed with 60% fructose diet; (4) metabolic syndrome + stone former group (MS + SF) (n = 10), 60% fructose diet and 0.75% EG in the drinking water. MS group showed a significant injury to renal function when hyperoxaluria was induced. It was demonstrated by a significant decrease of creatinine clearance (p < 0.001), with higher tubular damage (34.3%, CI 95% 23.9–44.7, p < 0.001), produced by deposition of crystals, and increased tubular synthesis of osteopontin as a response to tubular damage. Induction of hyperoxaluria in rats with MS causes severe morphological alterations with a significant impairment of renal function. This impairment is not produced in rats without MS. Therefore, this model can be useful for the study of the influence of MS in stone formation.



Similar content being viewed by others
References
Guize L, Pannier B, Thomas F, Bean K, Jego B, Benetos A (2008) Recent advances in metabolic syndrome and cardiovascular disease. Arch Cardiovasc Dis 101:577–583
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA et al (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: executive summary. Crit Pathw Cardiol. 4:198–203
Pannier B, Thomas F, Eschwege E, Bean K, Benetos A, Leocmach Y et al (2006) Cardiovascular risk markers associated with the metabolic syndrome in a large French population: the “SYMFONIE” study. Diabetes Metab. 32:467–474
Guize L, Thomas F, Pannier B, Bean K, Danchin N, Benetos A (2006) Metabolic syndrome: prevalence, risk factors and mortality in a French population of 62 000 subjects. Bull Acad Natl Med. 190:685–697 (discussion 697)
Bobulescu IA (2010) Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens 19:393–402
Unger RH (2002) Lipotoxic diseases. Annu Rev Med 53:319–336
Jonassen JA, Kohjimoto Y, Scheid CR, Schmidt M (2005) Oxalate toxicity in renal cells. Urol Res 33:329–339
West B, Luke A, Durazo-Arvizu RA, Cao G, Shoham D, Kramer H (2008) Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988-1994. Am J Kidney Dis 51:741–747
Rendina D, Mossetti G, De Filippo G, Benvenuto D, Vivona CL, Imbroinise A et al (2009) Association between metabolic syndrome and nephrolithiasis in an inpatient population in southern Italy: role of gender, hypertension and abdominal obesity. Nephrol Dial Transplant 24:900–906
Jeong IG, Kang T, Bang JK, Park J, Kim W, Hwang SS et al (2011) Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis 58:383–388
Chou YH, Su CM, Li CC, Liu CC, Liu ME, Wu WJ et al (2011) Difference in urinary stone components between obese and non-obese patients. Urol Res 39:283–287
Rofe AM, Bais R, Conyers RA (1986) The effect of dietary refined sugars and sugar alcohols on renal calcium oxalate deposition in ethylene glycol-treated rats. Food Chem Toxicol 24:397–403
Okamoto M, Kohjimoto Y, Iba A, Saji F, Hara I, Shigematsu T (2010) Calcium oxalate crystal deposition in metabolic syndrome model rat kidneys. Int J Urol 17:996–1003
Tran LT, Yuen VG, McNeill JH (2009) The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem 332:145–159
Pokrywczynska M, Flisinski M, Jundzill A, Krzyzanowska S, Brymora A, Deptula A et al (2014) Impact of fructose diet and renal failure on the function of pancreatic islets. Pancreas 43:801–808
Sanchez-Lozada LG, Tapia E, Jimenez A, Bautista P, Cristobal M, Nepomuceno T et al (2007) Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol 292:F423–F429
Khan SR (1997) Animal models of kidney stone formation: an analysis. World J Urol 15:236–243
Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CY (2004) Association of urinary pH with body weight in nephrolithiasis. Kidney Int 65:1422–1425
Coe FL, Strauss AL, Tembe V, Le Dun S (1980) Uric acid saturation in calcium nephrolithiasis. Kidney Int 17:662–668
Tiselius HG, Berg C, Fornander AM, Nilsson MA (1993) Effects of citrate on the different phases of calcium oxalate crystallization. Scanning Microsc. 7:381–389 (discussion 389)
Khan SR, Johnson JM, Peck AB, Cornelius JG, Glenton PA (2002) Expression of osteopontin in rat kidneys: induction during ethylene glycol induced calcium oxalate nephrolithiasis. J Urol 168:1173–1181
Acknowledgements
The Urological Society from Madrid, SUM Research Grant 2013 and Astellas Pharma España are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Urological Society from Madrid. Research Grant 2013 and Astellas Pharma España are acknowledged.
Conflict of interest
Sáenz-Medina J., Jorge E., Corbacho C., Santos M., Sánchez A., Soblechero P., Virumbrales E., Ramil E., Coronado M.J., Castillón I., Carballido J. declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Sáenz-Medina, J., Jorge, E., Corbacho, C. et al. Metabolic syndrome contributes to renal injury mediated by hyperoxaluria in a murine model of nephrolithiasis. Urolithiasis 46, 179–186 (2018). https://doi.org/10.1007/s00240-017-0979-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-017-0979-9