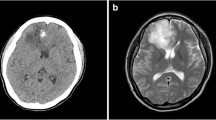
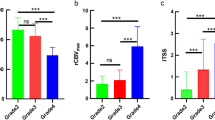
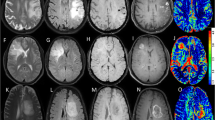
Abstract
Purpose
This retrospective study was performed on a 3T MRI to determine the unique conventional MR imaging and T1-weighted DCE-MRI features of oligodendroglioma and astrocytoma and investigate the utility of machine learning algorithms in their differentiation.
Methods
Histologically confirmed, 81 treatment-naïve patients were classified into two groups as per WHO 2016 classification: oligodendroglioma (n = 16; grade II, n = 25; grade III) and astrocytoma (n = 10; grade II, n = 30; grade III). The differences in tumor morphology characteristics were evaluated using Z-test. T1-weighted DCE-MRI data were analyzed using an in-house built MATLAB program. The mean 90th percentile of relative cerebral blood flow, relative cerebral blood volume corrected, volume transfer rate from plasma to extracellular extravascular space, and extravascular extracellular space volume values were evaluated using independent Student’s t test. Support vector machine (SVM) classifier was constructed to differentiate two groups across grade II, grade III, and grade II+III based on statistically significant features.
Results
Z-test signified only calcification among conventional MR features to categorize oligodendroglioma and astrocytoma across grade III and grade II+III tumors. No statistical significance was found in the perfusion parameters between two groups and its subtypes. SVM trained on calcification also provided moderate accuracy to differentiate oligodendroglioma from astrocytoma.
Conclusion
We conclude that conventional MR features except calcification and the quantitative T1-weighted DCE-MRI parameters fail to discriminate between oligodendroglioma and astrocytoma. The SVM could not further aid in their differentiation. The study also suggests that the presence of more than 50% T2-FLAIR mismatch may be considered as a more conclusive sign for differentiation of IDH mutant astrocytoma.





Similar content being viewed by others
References
Walsh KM, Ohgaki H, Wrensch MR (2016) Epidemiology. Handb Clin Neurol 134:3–18
Ostrom QT, Gittleman H, Liao P, et al. (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol 16: iv1-63.
Chinot O (2001) Chemotherapy for the treatment of oligodendroglial tumors. Semin Oncol 28:13–18
Perry A (2001) Oligodendroglial neoplasms: current concepts, misconceptions, and folklore. Adv Anat Pathol 8:183–199
Engelhard HH, Stelea A, Mundt A (2003) Oligodendroglioma and anaplastic oligodendroglioma: clinical features, treatment, and prognosis. Surg Neurol 60:443–456
Van den Bent M (2001) New perspectives for the diagnosis and treatment of oligodendroglioma. Expert Rev Anticancer Ther 1:348–356
Sasaki H, Zlatescu MC, Betensky RA, Johnk LB, Cutone AN, Cairncross JG, Louis DN (2002) Histopathological molecular genetic correlations in referral pathologist-diagnosed low-grade “oligodendroglioma”. J Neuropathol Exp Neurol 61:58–63
Cha S, Tihan T, Crawford F, Fischbein NJ, Chang S, Bollen A, Nelson SJ, Prados M, Berger MS, Dillon WP (2005) Differentiation of low grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 26:266–273
Smits M (2016) Imaging of oligodendroglioma. Br J Radiol 89:20150857
Patel SH, Poisson LM, Brat DJ, Zhou Y, Cooper L, Snuderl M, Thomas C, Franceschi AM, Griffith B, Flanders AE, Golfinos JG, Chi AS, Jain R (2017) T2-FLAIR mismatch, an imaging biomarker for IDH and 1p/19q status in lower grade gliomas: a TCGA/TCIA project. Clin Cancer Res 23:6078–6085
Yoon HJ, Ahn KJ, Lee S, Jang JH, Choi HS, Jung SL, Kim BS, Jeun SS, Hong YK (2017) Differential diagnosis of oligodendroglial and astrocytic tumors using imaging results: the added value of perfusion MR imaging. Neuroradiology 59:665–675
van den Bent MJ, Chang SM (2018) Grade II and III oligodendroglioma and astrocytoma. Neurol Clin 36:467–484
Lasocki A, Gaillard F, Gorelik A, Gonzales M (2018) MRI features can predict 1p/19q Status in intracranial gliomas. AJNR Am J Neuroradiol 39:687–692
Pomper MG, Port JD (2000) New techniques in MR imaging of brain tumors. Magn Reson Imaging Clin N Am 8:691–713
Spampinato MV, Smith JK, Kwock L, Ewend M, Grimme JD, Camacho DLA, Castillo M (2007) Cerebral blood volume measurements and proton MR spectroscopy in grading of oligodendroglial tumors. AJR Am J Roentgenol 188:204–212
Chawla S, Krejza J, Vossough A, Zhang Y, Kapoor GS, Wang S, O'Rourke DM, Melhem ER, Poptani H (2013) Differentiation between oligodendroglioma genotypes using dynamic susceptibility contrast perfusion-weighted imaging and proton MR spectroscopy. AJNR Am J Neuroradiol 34:1542–1549
Fellah S, Caudal D, De Paula AM et al (2013) Multimodal MR imaging (diffusion, perfusion, and spectroscopy): is it possible to distinguish oligodendroglial tumor grade and 1p/19q codeletion in the pretherapeutic diagnosis? AJNR Am J Neuroradiol 34:1326–1333
Lin Y, Xing Z, She D, Yang X, Zheng Y, Xiao Z, Wang X, Cao D (2017) IDH mutant and 1p/19q co-deleted oligodendrogliomas: tumor grade stratification using diffusion-, susceptibility-, and perfusion-weighted MRI. Neuroradiology 59:555–562
Law M, Young R, Babb J, Pollack E, Johnson G (2007) Histogram analysis versus region of interest analysis of dynamic susceptibility contrast perfusion MR imaging data in the grading of cerebral gliomas. AJNR Am J Neuroradiol 28:761–766
Law M, Yang S, Babb JS, Knopp EA, Golfinos JG, Zagzag D, Johnson G (2004) Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 25:746–755
Falk A, Fahlström M, Rostrup E, Berntsson S, Zetterling M, Morell A, Larsson HBW, Smits A, Larsson EM (2014) Discrimination between glioma grades II and III in suspected low-grade gliomas using dynamic contrast enhanced and dynamic susceptibility contrast perfusion MR imaging: a histogram analysis approach. Neuroradiology 56:1031–1038
Saini J, Gupta PK, Sahoo P, Singh A, Patir R, Ahlawat S, Beniwal M, Thennarasu K, Santosh V, Gupta RK (2018) Differentiation of grade II/III and grade IV glioma by combining “T1 contrast-enhanced brain perfusion imaging” and susceptibility-weighted quantitative imaging. Neuroradiology 60:43–50
Singh A, Haris M, Rathore D, Purwar A, Sarma M, Bayu G, Husain N, Rathore RKS, Gupta RK (2007) Quantification of physiological and hemodynamic indices using T1 dynamic contrast-enhanced MRI in intracranial mass lesions. J Magn Reson Imaging 26:871–880
Jung SC, Yeom JA, Kim JH, Ryoo I, Kim SC, Shin H, Lee AL, Yun TJ, Park CK, Sohn CH, Park SH, Choi SH (2014) Glioma: application of histogram analysis of pharmacokinetic parameters from T1- weighted dynamic contrast-enhanced MR imaging to tumor grading. AJNR Am J Neuroradiol 35:1103–1110
Arevalo-Perez J, Thomas AA, Kaley T, Lyo J, Peck KK, Holodny AI, Mellinghoff IK, Shi W, Zhang Z, Young RJ (2015) T1-weighted dynamic contrast-enhanced MRI as a noninvasive biomarker of epidermal growth factor receptor vIII status. AJNR Am J Neuroradiol 36:2256–2261
Kickingereder P, Sahm F, Wiestler B, Roethke M, Heiland S, Schlemmer HP, Wick W, von Deimling A, Bendszus M, Radbruch A (2014) Evaluation of microvascular permeability with dynamic contrast-enhanced MRI for the differentiation of primary CNS lymphoma and glioblastoma: radiologic-pathologic correlation. AJNR Am J Neuroradiol 35:1503–1508
Sahoo P, Rathore RKS, Awasthi R, Roy B, Verma S, Rathore D, Behari S, Husain M, Husain N, Pandey CM, Mohakud S, Gupta RK (2013) Sub compartmentalization of extracellular extravascular space (EES) into permeability and leaky space with local arterial input function (AIF) results in improved discrimination between high- and low-grade glioma using dynamic contrast-enhanced (DCE) MRI. J Magn Reson Imaging 38:677–688
Arevalo-Perez J, Kebede AA, Peck KK, Diamond E, Holodny AI, Rosenblum M, Rubel J, Gaal J, Hatzoglou V (2016) Dynamic contrast-enhanced MRI in low-grade versus anaplastic oligodendrogliomas. J Neuroimaging 26:366–371
Saini J, Gupta RK, Kumar M, Singh A, Saha I, Santosh V, Beniwal M, Kandavel T, Cauteren MV (2019) Comparative evaluation of cerebral gliomas using rCBV measurements during sequential acquisition of T1-perfusion and T2*-perfusion MRI. PLoS One. 14:e0215400
Leu K, Ott GA, Lai A, Nghiemphu PL, Pope WB, Yong WH, Liau LM, Cloughesy TF, Ellingson BM (2017) Perfusion and diffusion MRI signatures in histologic and genetic subtypes of WHO grade II-III diffuse gliomas. J Neuro oncol 134:177–188
Lee JY, Ahn KJ, Lee YS, Jang JH, Jung SL, Kim BS (2018) Differentiation of grade II and III oligodendrogliomas from grade II and III astrocytomas: a histogram analysis of perfusion parameters derived from dynamic contrast-enhanced (DCE) and dynamic susceptibility contrast (DSC) MRI. Acta Radiol. 59:723–731
Blumenthal DT, Artzi M, Liberman G, Bokstein F, Aizenstein O, Ben Bashat D (2017) Classification of high-grade glioma into tumor and non-tumor components using support vector machine. AJNR Am J Neuroradiol 38:908–914
Citak-Er F, Firat Z, Kovanlikaya I, Ture U, Ozturk-Isik E (2018) Machine-learning in grading of gliomas based on multi-parametric magnetic resonance imaging at 3T. Comput Biol Med 99:154–160
Papp L, Pötsch N, Grahovac M, Schmidbauer V, Woehrer A, Preusser M, Mitterhauser M, Kiesel B, Wadsak W, Beyer T, Hacker M, Traub-Weidinger T (2018) Glioma survival prediction with combined analysis of in vivo (11)C-MET PET features, ex vivo features, and patient features by supervised machine learning. J Nucl Med 59:892–899
Zhang J, Yu H, Qian X, Liu K, Tan H, Yang T, Wang M, Li KC, Chan MD, Debinski W, Paulsson A, Wang G, Zhou X (2016) Pseudo progression identification of glioblastoma with dictionary learning. Computers in biology and medicine 73:94–101
Kunimatsu A, Kunimatsu N, Yasaka K, Akai H, Kamiya K, Watadani T, Mori H, Abe O (2019) Machine learning-based texture analysis of contrast-enhanced MR imaging to differentiate between glioblastoma and primary central nervous system lymphoma. Magn Reson Med Sci 18:44–52
Fan Y, Chen C, Zhao F, Tian Z, Wang J, Ma X, Xu J (2019) Radiomics-based machine learning technology enables better differentiation between glioblastoma and anaplastic oligodendroglioma. Frontiers in oncology 9:1164
Zhang Y, Chen C, Cheng Y, Teng Y, Guo W, Xu H, Ou X, Wang J, Li H, Ma X, Xu J (2019) Ability of radiomics in differentiation of anaplastic oligodendroglioma from atypical low-grade oligodendroglioma using machine-learning approach. Frontiers in oncology 9:1371
Sengupta A, Ramaniharan AK, Gupta RK, Agarwal S, Singh A (2019) Glioma grading using a machine-learning framework based on optimized features obtained from T1 perfusion MRI and volumes of tumor components. J Magn Reson Imaging. 50:1295–1306
Sengupta A, Agarwal S, Gupta PK, Ahlawat S, Patir R, Gupta RK, Singh A (2018) On differentiation between vasogenic edema and non-enhancing tumor in high-grade glioma patients using support vector machine classifier based upon pre and post surgery MRI images. Eur J Radiol 106:199–208
Sahoo P, Gupta RK, Gupta PK, Awasthi A, Pandey CM, Gupta M, Patir R, Vaishya S, Ahlawat S, Saha I (2017) Diagnostic accuracy of automatic normalization of CBV in glioma grading using T1- weighted DCE-MRI. Magn Reson Imaging 44:32–37
Sriraam N, Raghu S (2017) Classification of focal and non focal epileptic seizures using multi-features and SVM classifier. J Med Syst. Sep 41:160. https://doi.org/10.1007/s10916-017-0800-x
Ohsaki M, Wang P, Matsuda K, Katagiri S, Watanabe H, Ralescu A (2017) Confusion-matrix-based kernel logistic regression for imbalanced data classification. IEEE Trans. Knowl. Data Eng. 29:1806–1819
Deguchi S, Oishi T, Mitsuya K, Kakuda Y, Endo M, Sugino T, Hayashi N (2020) Clinicopathological analysis of T2-FLAIR mismatch sign in lower-grade gliomas. Scientific reports 10(1):10113
Narang J, Jain R, Scarpace L, Saksena S, Schultz LR, Rock JP, Rosenblum M, Patel SC, Mikkelsen T (2011) Tumor vascular leakiness and blood volume estimates in oligodendrogliomas using perfusion CT: an analysis of perfusion parameters helping further characterize genetic subtypes as well as differentiate from astroglial tumors. J Neuro-Oncol 102:287–293
Johnson DR, Kaufmann TJ, Patel SH, Chi AS, Snuderl M, Jain R (2019) There is an exception to every rule-T2-FLAIR mismatch sign in gliomas. Neuroradiology 61:225–227
Goyal A, Yolcu YU, Goyal A et al. (2019) The T2-FLAIR-mismatch sign as an imaging biomarker for IDH and 1p/19q status in diffuse low-grade gliomas: a systematic review with a Bayesian approach to evaluation of diagnostic test performance. Neurosurg Focus. 1;47(6):E13.
Foltyn M, Nieto Taborda KN, Neuberger U, et al. (2020) T2/FLAIR-mismatch sign for noninvasive detection of IDH-mutant 1p/19q non-codeleted gliomas: validity and pathophysiology. Neurooncol Adv 10;2(1):vdaa 004.
Corell A, Ferreyra Vega S, Hoefling N, Carstam L, Smits A, Olsson Bontell T, Björkman-Burtscher IM, Carén H, Jakola AS (2020) The clinical significance of the T2-FLAIR mismatch sign in grade II and III gliomas: a population-based study. BMC Cancer 20:450
Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, Coons SW, Nakaji P, Yeh RF, Debbins J, Heiserman JE (2009) Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 30:552–558
Heo YJ, Kim HS, Park JE, et al. (2015) Uninterpretable dynamic susceptibility contrast-enhanced perfusion MR images in patients with post-treatment glioblastomas: cross-validation of alternative imaging options. PLoS One 21;10(8):e0136380.
Anzalone N, Castellano A, Cadioli M et al. (2018) Brain gliomas: multicenter standardized assessment of dynamic contrast-enhanced and dynamic susceptibility contrast MR images. mnjbuhnbvdcxmjhxjhxcj0068
Funding
This study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
For this type of retrospective study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(JPG 91 kb)
Rights and permissions
About this article
Cite this article
Gupta, M., Gupta, A., Yadav, V. et al. Comparative evaluation of intracranial oligodendroglioma and astrocytoma of similar grades using conventional and T1-weighted DCE-MRI. Neuroradiology 63, 1227–1239 (2021). https://doi.org/10.1007/s00234-021-02636-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02636-8