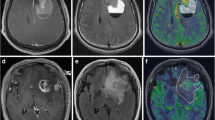
Abstract
The purpose of this study was to determine the usefulness of perfusion CT (PCT) parameters particularly blood volume and neovascular permeability estimates (permeability surface area-product, PS) in the evaluation of oligodendrogliomas (OG), correlation with genetic subtypes of OGs (with or without loss of heterozygosity/LOH on 1p/19q) as well as comparison of perfusion parameters of OGs with astroglial tumors. Pre-operative PCT done in 21 patients with OGs was retrospectively correlated with our previously published PCT data for 32 patients with astroglial neoplasms (Jain R et al., AJNR Am J Neuroradiol 29:694–700, 2008). All OGs were also analyzed for genetic subtypes of with or without LOH. PCT parameters PS and cerebral blood volume (CBV) were obtained for the entire lesion and a statistical analysis done to correlate various histopathological variants. Low grade OGs (n = 13) showed slightly lower CBV (1.42 vs. 1.72 ml/100 g; P value 0.391) and PS (0.56 vs. 0.95 ml/100 g/min; P value 0.099) as compared to high grade OGs (n = 8), though not statistically significant. LOH positive OGs (n = 13) showed higher mean CBV (1.59 vs. 1.45; P value 0.712) and slightly lower PS (0.68 vs. 0.75; P value 0.718) as compared to LOH negative OGs (n = 8), although not statistically significant. Low grade OGs (n = 13) showed higher mean CBV 1.42 ml/100 g as compared to low grade astroglial tumors (n = 8) 0.95 ml/100 g (P value = 0.08), however no statistically significant difference was noted for PS (0.56 vs. 0.52 ml/100 g/min, P value 0.695). Statistically significant differences were observed in CBV and PS values of high grade OGs and high grade astroglial tumors with the high grade glial tumors showing higher mean CBV (2.79 vs. 1.72; P value 0.03) as well as higher PS (2.37 vs. 0.95; P value < 0.01), however this difference was not significant if only comparing grade III OGs with grade III astroglial tumors. PCT perfusion parameters including PS values do not help grade OGs despite showing a trend for higher CBV and PS in higher grade OGs. Similarly LOH positive OGs also showed slightly higher CBV, but again failed to reach any statistically significant level. Low grade OGs showed slightly higher CBV as compared to low grade astroglial tumors, whereas higher grade OGs showed significantly lower PS values as compared to higher grade astroglial tumors despite showing high CBV.




Similar content being viewed by others
References
Behin A, Hoang-Xian K, Carpentier AF, Delattre JY (2003) Primary brain tumors in adults. Lancet 361:323–331. doi:10.1016/s0140-6736(03)12328-8
Fortin D, Cairncross GJ, Hammond RR (1999) Oligodendroglioma: an appraisal of recent data pertaining to diagnosis and treatment. Neurosurgery 45:1279–1291
Engelhard HH, Stelea A, Cochran EJ (2002) Oligodendroglioma: pathology and molecular biology. Surg Neurol 58:111–117
Engelhard HH, Stelea A, Mundt A (2003) Oligodendroglioma and anaplastic oligodendroglioma: clinical features, treatment, and prognosis. Surg Neurol 60:443–456
Cairncross JG, Macdonald DR (1988) Successful chemotherapy for recurrent malignant oligodendroglioma. Ann Neurol 23:360–364. doi:10.1002/ana.410230408
Coons SW, Johnson PC, Pearl DK (1997) The prognostic significance of Ki-67 labeling indices for oligodendrogliomas. Neurosurgery 41:878–884
Van den Bent MJ, Looijenga LH, Langenberg K, Dinjens W, Graveland W, Uytdewilligen L, Sillevis Smitt PA, Jenkins RB, Kros JM (2003) Chromosomal anomalies in oligodendroglial tumors are correlated with clinical features. Cancer 97:1276–1284. doi:10.1002/cncr.11187
Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y, Ramsay DA, Louis DN (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90:1473–1479
Shaw EG, Scheithauer BW, O’Fallon JR, Tazelaar HD, Davis DH (1992) Oligodendrogliomas: the Mayo Clinic experience. J Neurosurg 76:428–434. doi:10.3171/jns.1992.76.3.0428
Aronen HJ, Gazit IE, Louis DN, Buchbinder BR, Pardo FS, Weisskoff RM, Harsh GR, Cosgrove GR, Halpern EF, Hochberg FH et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Lev MH, Ozsunar Y, Henson JW, Rasheed AA, Barest GD, Harsh GRt, Fitzek MM, Chiocca EA, Rabinov JD, Csavoy AN, Rosen BR, Hochberg FH, Schaefer PW, Gonzalez RG (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas. AJNR Am J Neuroradiol 25:214–221
Lev MH, Rosen BR (1999) Clinical applications of intracranial perfusion MR imaging. Neuroimaging Clin N Am 9:309–331
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Hakyemez B, Erdogan C, Ercan I, Ergin N, Uysal S, Atahan S (2005) High-grade and low-grade gliomas: differentiation by using perfusion MR imaging. Clin Radiol 60:493–502. doi:10.1016/j.crad.2004.09.009
Rijpkema M, Schuuring J, van der Meulen Y, van der Graaf M, Bernsen H, Boerman R, van der Kogel A, Heerschap A (2003) Characterization of oligodendrogliomas using short echo time 1H MR spectroscopic imaging. NMR Biomed 16:12–18. doi:10.1002/nbm.807
Ellika SK, Jain R, Patel SC, Scarpace L, Schultz LR, Rock JP, Mikkelsen T (2007) Role of perfusion CT in glioma grading and comparison with conventional MR imaging features. AJNR Am J Neuroradiol 28:1981–1987. doi:10.3174/ajnr.A0688
Cha S, Tihan T, Crawford F, Fischbein NJ, Chang S, Bollen A, Nelson SJ, Prados M, Berger MS, Dillon WP (2005) Differentiation of low grade oligodendrogliomas from low grade astrocytomas using quantitative blood volume measurements derived from dynamic susceptibility contrast enhanced MR imaging. AJNR Am J Neuroradiol 26:266–273
Jain R, Ellika SK, Scarpace L, Schultz LR, Rock JP, Gutierrez J, Patel SC, Ewing J, Mikkelsen T (2008) Quantitative estimation of permeability surface-area product in astroglial brain tumors using perfusion CT and correlation with histopathologic grade. AJNR Am J Neuroradiol 29:694–700. doi:10.3174/ajnr.A0899
Jain R, Scarpace L, Ellika S, Schultz LR, Rock JP, Rosenblum ML, Patel SC, Lee TY, Mikkelsen T (2007) First-pass perfusion computed tomography: initial experience in differentiating recurrent brain tumors from radiation effects and radiation necrosis. Neurosurgery 61:778–786. doi:10.1227/01.neu.0000298906.48388.26
Pech IV, Peterson K, Cairncross JG (1998) Chemotherapy for brain tumors. Oncology (Williston Park) 12:537–543
Friedman HS, Lovell S, Rasheed K, Friedman AH (1998) Treatment of adults with progressive oligodendroglioma with carboplatin (CBDCA): preliminary results. Writing Committee for the Brain Tumor Center at Duke. Med Pediatr Oncol 31:16–18
Soffietti R, Ruda R, Bradac GB, Schiffer D (1998) PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery 43:1066–1073
Chinot O (2001) Chemotherapy for the treatment of oligodendroglial tumors. Semin Oncol 28:13–18
Christov C, Adle-Biassette H, Le Guerinel C, Natchev S, Gherardi RK (1998) Immunohistochemical detection of vascular endothelial growth factor (VEGF) in the vasculature of oligodendrogliomas. Neuropathol Appl Neurobiol 24:29–35
Pietsch T, Valter MM, Wolf HK, von Deimling A, Huang HJ, Cavenee WK, Wiestler OD (1997) Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathol 93:109–117
Maia AC Jr, Malheiros SM, da Rocha AJ, da Silva CJ, Gabbai AA, Ferraz FA, Stavale JN (2005) MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. AJNR Am J Neuroradiol 26:777–783
Netto GC, Bleil CB, Hilbig A, Coutinho LM (2008) Immunohistochemical evaluation of the microvascular density through the expression of TGF-b (CD 105/endoglin) and CD 34 receptors and expression of the vascular endothelial growth factor (VEGF) in oligodendrogliomas. Neuropathology 28:17–23. doi:10.1111/j.1440-1789.2007.00825.x
Law M, Yang S, Babb JS, Knopp EA, Golfinos JG, Zagzag D, Johnson G (2004) Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 25:746–755
Jain R, Gutierrez J, Narang J, Rock J P, Scarpace L, Schultz LR, Lemke N, Patel SC, Mikkelsen T (in press) In vivo correlation of tumor blood volume and permeability with histological and molecular angiogenic markers in gliomas. Unpublished data
Xu M, See SJ, Ng WH, Arul E, Back MF, Yeo TT, Lim CC (2005) Comparison of magnetic resonance spectroscopy and perfusion-weighted imaging in presurgical grading of oligodendroglial tumors. Neurosurgery 56:919–926
Christov C, Gherardi RK (1999) Vascular endothelial growth factor (VEGF) likely contributes to oligodendroglioma angiogenesis. Acta Neuropathol 97(4):429–432
Varlet P, Guillamo JS, Nataf F, Koziak M, Beuvon F, Daumas-Duport C (2000) Vascular endothelial growth factor expression in oligodendrogliomas: a correlative study with Sainte-Anne malignancy grade, growth fraction and patient survival. Neuropathol Appl Neurobiol 26:379–389
Spampinato MV, Smith JK, Kwock L, Ewend M, Grimme JD, Camacho DL, Castillo M (2007) Cerebral blood volume measurements and proton MR spectroscopy in grading of oligodendroglial tumors. AJR Am J Roentgenol 18:204–212. doi:10.2214/ajr.05.1177
Whitmore RG, Krejza J, Huse J, Woo JH, Bloom S, Lopinto J, Wolf RL, Judy K, Rosenfeld MR, Biegel JA, Melhem ER, O’Rourke DM (2007) Prediction of oligodendroglial tumor subtype and grade using perfusion weighted magnetic resonance imaging. J Neurosurg 107:600–609. doi:10.3171/jns-07/09/0600
Warnke PC, Berlis A, Ostertag C (1998) Physiological characterization of malignant oligodendrogliomas responding to PCV chemotherapy. J Neurosurg 88:399A
Jager HR, Waldman AD, Benton C, Fox N, Rees J (2005) Differential chemosensitivity of tumor components in a malignant oligodendroglioma: assessment with diffusion-weighted, perfusion-weighted, and serial volumetric MR imaging. AJNR Am J Neuroradiol 26:274–278
Godfraind C, Rousseau E, Ruchoux MM, Scaravilli F, Vikkula M (2003) Tumour necrosis and microvascular proliferation are associated with 9p deletion and CDKN2A alterations in 1p/19q deleted oligodendrogliomas. Neuropathol Appl Neurobiol 29:462–464
Jenkinson MD, Smith TS, Joyce KA, Fildes D, Broome J, du Plessis DG, Haylock B, Husband DJ, Warnke PC, Walker C (2006) Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumors. Neuroradiology 48:703–713
Kapoor GS, Gocke TA, Chawla S, Whitmore RG, Nabavizadeh A, Krejza J, Lopinto J, Plaum J, Maloney-Wilensky E, Poptani H, Melhem ER, Judy KD, O’Rourke DM (2009) Magnetic resonance perfusion-weighted imaging defines angiogenic subtypes of oligodendroglioma according to 1p19q and EGFR status. J Neurooncol 92:373–386. doi:10.1007/s11060-009-9880-x
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Narang, J., Jain, R., Scarpace, L. et al. Tumor vascular leakiness and blood volume estimates in oligodendrogliomas using perfusion CT: an analysis of perfusion parameters helping further characterize genetic subtypes as well as differentiate from astroglial tumors. J Neurooncol 102, 287–293 (2011). https://doi.org/10.1007/s11060-010-0317-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0317-3