Abstract
Purpose
To investigate the clinical utility of pointwise encoding time reduction with radial acquisition in subtraction-based magnetic resonance angiography (PETRA-MRA) and time-of-flight magnetic resonance angiography (TOF-MRA) to evaluate saccular unruptured intracranial aneurysms (UIAs).
Methods
A total of 49 patients with 54 TOF-MRA–identified saccular UIAs were enrolled. The morphologic parameters, contrast-to-noise-ratios (CNRs), and sharpness of aneurysms were measured using PETRA-MRA and TOF-MRA. Two radiologists independently evaluated subjective image scores, focusing on aneurysm signal homogeneities and sharpness depictions using a 4-point scale: 4, excellent; 3, good; 2, poor; 1, not assessable. PETRA-MRA and TOF-MRA acoustic noises were measured.
Results
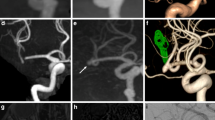
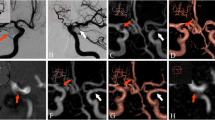
All aneurysms were detected with PETRA-MRA. The morphologic parameters of 15 patients evaluated with PETRA-MRA were more closely correlated with those receiving computed tomography angiography over those receiving TOF-MRA. No significant differences between PETRA-MRA and TOF-MRA parameters were seen in the 54 UIAs (p > 0.10), excluding those with inflow angles (p < 0.05). In four patients with inflow angles on PETRA-MRA, the angles were more closely related to those of digital subtraction angiography than those of TOF-MRA. CNRs between TOF-MRA and PETRA-MRA were comparable (p = 0.068), and PETRA-MRA sharpness values and subjective image scores were significantly higher than those of TOF-MRA (p < 0.001). Inter-observer agreements were excellent for both PETRA-MRA and TOF-MRA (intraclass correlation coefficients were 0.90 and 0.97, respectively). The acoustic noise levels of PETRA-MRA were much lower than those of TOF-MRA (59 vs.73 dB, p < 0.01).
Conclusions
PETRA-MRA, with better visualization of aneurysms and lower acoustic noise levels than TOF-MRA, showed a superior diagnostic performance for depicting saccular UIAs.





Similar content being viewed by others
References
Lu HT, Tan HQ, Gu BX, Wu-Wang, Li MH (2013) Risk factors for multiple intracranial aneurysms rupture: a retrospective study. Clin Neurol Neurosurg 115:690–694. https://doi.org/10.1016/j.clineuro.2012.08.011
Suarez JI, Tarr RW, Selman WR (2006) Aneurysmal subarachnoid hemorrhage. N Engl J Med 354:387–396. https://doi.org/10.1056/NEJMra052732
van Gijn J, Rinkel GJ (2001) Subarachnoid haemorrhage: diagnosis, causes and management. Brain 124:249–278. https://doi.org/10.1093/brain/124.2.249
Juvela S, Porras M, Poussa K (2000) Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg 93:379–387. https://doi.org/10.3171/jns.2000.93.3.0379
Kimura H, Hayashi K, Taniguchi M, Hosoda K, Fujita A, Seta T, Tomiyama A, Kohmura E (2019) Detection of hemodynamic characteristics before growth in growing cerebral aneurysms by analyzing time-of-flight magnetic resonance angiography images alone: preliminary results. World Neurosurg 122:e1439–e1448. https://doi.org/10.1016/j.wneu.2018.11.081
Vlak MH, Algra A, Brandenburg R et al (2011) Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 10:626–636. https://doi.org/10.1016/S1474-4422(11)70109-0
Cirillo M, Scomazzoni F, Cirillo L, Cadioli M, Simionato F, Iadanza A, Kirchin M, Righi C, Anzalone N (2013) Comparison of 3D TOF-MRA and 3D CE-MRA at 3T for imaging of intracranial aneurysms. Eur J Radiol 82:e853–e859. https://doi.org/10.1016/j.ejrad.2013.08.052
van Rooij WJ, Sprengers ME, de Gast AN, Peluso JPP, Sluzewski M (2008) 3D rotational angiography: the new gold standard in the detection of additional intracranial aneurysms. AJNR Am J Neuroradiol 29:976–979. https://doi.org/10.3174/ajnr.A0964
Katzberg RW (1997) Urography into the 21st century: new contrast media, renal handling, imaging characteristics, and nephrotoxicity. Radiology 204:297–312. https://doi.org/10.1148/radiology.204.2.9240511
Heiserman JE, Dean BL, Hodak JA et al (1994) Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol 15:1401–1407
Thompson BG, Brown RD, Amin-Hanjani S et al (2015) Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46:2368–2400. https://doi.org/10.1161/STR.0000000000000070
Stivaros SM, Harris JN, Adams W, Jackson A (2009) Does black blood MRA have a role in the assessment of intracerebral aneurysms? Eur Radiol 19:184–192. https://doi.org/10.1007/s00330-008-1127-2
Nael K, Villablanca JP, Saleh R, Pope W, Nael A, Laub G, Finn JP (2006) Contrast-enhanced MR angiography at 3T in the evaluation of intracranial aneurysms: a comparison with time-of-flight MR angiography. AJNR Am J Neuroradiol 27:2118–2121
Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE (2007) Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 242:647–649. https://doi.org/10.1148/radiol.2423061640
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782. https://doi.org/10.1148/radiol.15150025
Togao O, Hiwatashi A, Yamashita K, Momosaka D, Obara M, Nishimura A, Arimura K, Hata N, Iihara K, van Cauteren M, Honda H (2019) Acceleration-selective arterial spin labeling MR angiography for visualization of brain arteriovenous malformations. Neuroradiology 61:979–989. https://doi.org/10.1007/s00234-019-02217-w
Togao O, Hiwatashi A, Obara M, Yamashita K, Kikuchi K, Kamei R, Nishimura A, Arimura K, Yoshimoto K, Iihara K, van Cauteren M, Honda H (2017) Acceleration-selective arterial spin-labeling MR angiography used to visualize distal cerebral arteries and collateral vessels in moyamoya disease. Radiology 286:611–621. https://doi.org/10.1148/radiol.2017162279
Saloner D (1995) The AAPM/RSNA physics tutorial for residents. An introduction to MR angiography. RadioGraphics 15:453–465. https://doi.org/10.1148/radiographics.15.2.7761648
Patel MR, Klufas RA, Kim D, Edelman RR, Kent KC (1994) MR angiography of the carotid bifurcation: artifacts and limitations. Am J Roentgenol 162:1431–1437. https://doi.org/10.2214/ajr.162.6.8192013
Horikoshi T, Fukamachi A, Nishi H, Fukasawa I (1994) Detection of intracranial aneurysms by three-dimensional time-of-flight magnetic resonance angiography. Neuroradiology 36:203–207. https://doi.org/10.1007/bf00588131
Hacein-Bey L, Provenzale JM (2011) Current imaging assessment and treatment of intracranial aneurysms. Am J Roentgenol 196:32–44. https://doi.org/10.2214/AJR.10.5329
Pandey S, Hakky M, Kwak E, Jara H, Geyer CA, Erbay SH (2013) Application of basic principles of physics to head and neck MR angiography: troubleshooting for artifacts. RadioGraphics 33:E113–E123. https://doi.org/10.1148/rg.333125148
Lin Z, Zhang X, Guo L, Wang K, Jiang Y, Hu X, Huang Y, Wei J, Ma S, Liu Y, Zhu L, Zhuo Z, Liu J, Wang X (2019) Clinical feasibility study of 3D intracranial magnetic resonance angiography using compressed sensing. J Magn Reson Imaging 50:1843–1851. https://doi.org/10.1002/jmri.26752
Fushimi Y, Okada T, Kikuchi T, Yamamoto A, Okada T, Yamamoto T, Schmidt M, Yoshida K, Miyamoto S, Togashi K (2017) Clinical evaluation of time-of-flight MR angiography with sparse undersampling and iterative reconstruction for cerebral aneurysms. NMR Biomed 30(11):e3774. https://doi.org/10.1002/nbm.3774
Grodzki DM, Jakob PM, Heismann B (2012) Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med 67:510–518. https://doi.org/10.1002/mrm.23017
Natsuaki Y, Bi X, Grodzki DM et al (2015) PETRA qMRA: towards zero-flow dephasing intracranial non-contrast MR angiography. In: Proceedings of the 23rd Annual Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine
Natsuaki Y, Grimm R, Bi X et al (2016) PETRA quiet MRA: improved robustness with 3D elastic cross-registration. In: Proceedings of the 24th Annual Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine
Okuchi S, Fushimi Y, Okada T et al (2016) The pointwise encoding time reduction with radial acquisition (PETRA) sequence: visualization of intracranial arteries and facial nerve canals. In: Proceedings of the 24th Annual Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine
Heo YJ, Jeong HW, Baek JW, Kim ST, Jeong YG, Lee JY, Jin SC (2019) Pointwise encoding time reduction with radial acquisition with subtraction-based MRA during the follow-up of stent-assisted coil embolization of anterior circulation aneurysms. AJNR Am J Neuroradiol 40:815–819. https://doi.org/10.3174/ajnr.A6035
Ratering D, Baltes C, Dörries C, Rudin M (2010) Accelerated cardiovascular magnetic resonance of the mouse heart using self-gated parallel imaging strategies does not compromise accuracy of structural and functional measures. J Cardiovasc Magn Reson 12:43. https://doi.org/10.1186/1532-429X-12-43
Larson AC, Kellman P, Arai A, Hirsch GA, McVeigh E, Li D, Simonetti OP (2005) Preliminary investigation of respiratory self-gating for free-breathing segmented cine MRI. Magn Reson Med 53:159–168. https://doi.org/10.1002/mrm.20331
Shang S, Ye J, Luo X et al (2017) Follow-up assessment of coiled intracranial aneurysms using zTE MRA as compared with TOF MRA: a preliminary image quality study. Eur Radiol 27:4271–4280. https://doi.org/10.1007/s00330-017-4794-z
Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH, Hopkins LN, Meng H (2008) Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery 63:185–197. https://doi.org/10.1227/01.NEU.0000316847.64140.81
Baharoglu MI, Schirmer CM, Hoit DA, Gao BL, Malek AM (2010) Aneurysm inflow-angle as a discriminant for rupture in sidewall cerebral aneurysms: morphometric and computational fluid dynamic analysis. Stroke 41:1423–1430. https://doi.org/10.1161/STROKEAHA.109.570770
Wermer MJH, van der Schaaf IC, Algra A, Rinkel GJE (2007) Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke 38:1404–1410. https://doi.org/10.1161/01.STR.0000260955.51401.cd
Zheng Y, Xu F, Ren J, Xu Q, Liu Y, Tian Y, Leng B (2016) Assessment of intracranial aneurysm rupture based on morphology parameters and anatomical locations. J NeuroIntervent Surg 8:1240–1246. https://doi.org/10.1136/neurintsurg-2015-012112
Meng H, Tutino VM, Xiang J, Siddiqui A (2014) High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol 35:1254–1262. https://doi.org/10.3174/ajnr.A3558
Al-Kwifi O, Emery DJ, Wilman AH (2002) Vessel contrast at three tesla in time-of-flight magnetic resonance angiography of the intracranial and carotid arteries. Magn Reson Imaging 20:181–187. https://doi.org/10.1016/S0730-725X(02)00486-1
Nielsen HT, Gold GE, Olcott EW et al (1999) Ultra-short echo-time 2D time-of-flight MR angiography using a half-pulse excitation. Magn Reson Med 41:591–599. https://doi.org/10.1002/(sici)1522-2594(199903)41:3<591::aid-mrm23>3.0.co;2-r
McJury M, Shellock FG (2000) Auditory noise associated with MR procedures: a review. J Magn Reson Imaging 12:37–45. https://doi.org/10.1002/1522-2586(200007)12:1<37::AID-JMRI5>3.0.CO;2-I
Ida M, Wakayama T, Nielsen ML, Abe T, Grodzki DM (2015) Quiet T1-weighted imaging using PETRA: initial clinical evaluation in intracranial tumor patients: quiet T1-weighted imaging using PETRA. J Magn Reson Imaging 41:447–453. https://doi.org/10.1002/jmri.24575
Aida N, Niwa T, Fujii Y, Nozawa K, Enokizono M, Murata K, Obata T (2016) Quiet T1-weighted pointwise encoding time reduction with radial acquisition for assessing myelination in the pediatric brain. Am J Neuroradiol 37:1528–1534. https://doi.org/10.3174/ajnr.A4747
Dournes G, Grodzki D, Macey J, Girodet PO, Fayon M, Chateil JF, Montaudon M, Berger P, Laurent F (2015) Quiet submillimeter MR imaging of the lung is feasible with a PETRA sequence at 1.5 T. Radiology 276:258–265. https://doi.org/10.1148/radiol.15141655
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, Q., Liu, DX., Zhang, XY. et al. Pointwise encoding time reduction with radial acquisition in subtraction-based magnetic resonance angiography to assess saccular unruptured intracranial aneurysms at 3 Tesla. Neuroradiology 63, 189–199 (2021). https://doi.org/10.1007/s00234-020-02512-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02512-x