Abstract
For a long time, the physiological activity of TRP ion channels and the response to various stimuli have been the focus of attention, and the physiological functions mediated by ion channels have subtle links with the occurrence of various diseases. Our group has been engaged in the study of ion channels. In recent years, the report rate of TRPA1, the only member of the TRPA subfamily in the newly described TRP channel, has been very high. TRPA1 channels are not only abundantly expressed in peptidergic nociceptors but are also found in many nonneuronal cell types and tissues, and through the regulation of Ca2+ influx, various neuropeptides and signaling pathways are involved in the regulation of nerves, respiration, circulation, and various diseases and inflammation throughout the body. In this review, we mainly summarize the effects of TRPA1 on various systems in the body, which not only allows us to have a more systematic and comprehensive understanding of TRPA1 but also facilitates more in-depth research on it in the future.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
TRPA1 introduction
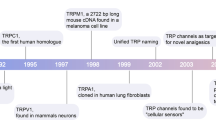
The TRP gene was first identified in Drosophila melanogaster in the late 1960s (Li 2017). The first human homolog was reported in 1995 (Li 2017). There are seven families of TRPs: TRPC, TRPV, TRPM, TRPA, TRPP, TRPML, and TRPN (Li 2017). TRP channels often affect cellular function by regulating protein expression levels, membrane excitability, and intracellular calcium levels, as cardiac and neuronal disorders are associated with aberrant TRP function (Souza Monteiro de Araúja et al. 2020a).
The ankyrin repeat channel TRPA1, a member of the TRP family, was first cloned in 1999, and in recent years has received much attention owing to its functional diversity as a signal sensor of stimuli and cell damage and its important role in many different diseases (Nilius et al. 2012). The development of various functions is inseparable from its special structure (Fig. 1), such as the elongated ankyrin repeat domain (ARD 14–18), which controls protein‒protein interactions, as well as channel insertion and regulation in the plasma membrane. In addition, it is much more permeable to Ca2+ than other TRPs (Souza Monteiro de Araújo et al. 2020b). From the cytoplasmic point of view, TRPA1 is tightly regulated by Ca2+ (Sura et al. 2012). It has been proposed that an EF-hand located within the N-terminus of TRPA1 mediates Ca2+ activation (Doerner et al. 2007; Zurborg et al. 2007), another putative Ca2+-binding domain is formed by residues E1077, D1080, D1081, and D1082 in the distal COOH-terminal region, the conserved acidic motif at the C-terminal is actively involved in the regulation of TRPA1 by Ca2+ (Sura et al. 2012). A highly conserved structural motif in TRPA1 is a key site for intracellular Ca2+ elevation caused by Ca2+ storage (Hu et al. 2021). TRPA1 can induce apoptosis of cardiomyocytes, oligodendrocytes, and hippocampal neurons by regulating Ca2+ concentration, and can also affect Ca2+-dependent signaling pathways, pain perception, and responses to environmental stimuli and irritating compounds (Earley 2012; Hu et al. 2021). This channel was initially found to be expressed in sensory neurons of the dorsal root ganglion (DRG), trigeminal ganglia, and tubercle ganglia, and later researchers gradually discovered that it is expressed in nonneuronal cells (Fig. 2) such as alveoli. It was expressed in Schwann cells, epithelial cells, cardiac fibroblasts (CF), pancreatic beta cells, enterochromaffin cells, T-cells, and 105 calcitonin gene-related peptide (CGRP)- and IB4-positive neurons. In these cells, it can affect a wide range of physiological processes, mainly through the effect of Ca2+ influx, and the regulation of oxidative stress and neuronal peptides release (SP, bradykinin, CGRP, etc.) (De Logu et al. 2017; Jha et al. 2015; Meents et al. 2019; Nilius et al. 2012; Wang et al. 2019b).
Expression of TRPA1 in the nervous, respiratory, cardiovascular, digestive, and urinary systems. Nervous system mainly expressed in small diameter C or Aδ fibers of sensory ganglia, including DRG, 99 trigeminal, and tubercle ganglia. Respiratory System expressed in trachea, bronchi, and alveolar epithelial cells. Circulatory system expressed in vascular endothelium, cardiomyocytes, and cardiac fibroblasts. Digestive system expressed in enterochromaffin cells in the gut, pancreatic beta cells. Urinary system expressed in renal tubular epithelial cells, urothelial, and smooth muscle cells in the bladder mucosa. Skin expressed in melanocytes
TRPA1-Related Ligands
TRPA1 Agonists
A variety of compounds activate TRPA1 channels, which can be broadly divided into two categories: electrophilic activators and nonelectrophilic activators. Furthermore, TRPA1 agonists can be classified as exogenous or endogenous (Strassmaier and Bakthavatchalam 2011). Electrophilic endogenous TRPA1 agonists include acrolein, reactive oxygen species, while exogenous agonists of TRPA1 include electrophiles such as allyl isothiocyanate (AITC), cinnamaldehyde (CA), and allicin. Although structurally dissimilar, they exert their activity by covalently modifying the intracellular N-terminal cysteine residue of TRPA1. TRPA1 can also be activated by noncovalently modified compounds (Skerratt 2017). Reactive and nonreactive compounds may activate TRPA1 by binding to different sites on the channel (Strassmaier and Bakthavatchalam 2011) and have different effects on a variety of diseases; for example, CA regulates blood sugar through TRPA1-ghrelin and other pathways (Zhu et al. 2017). In recent years, it has been suggested that CA may have a protective effect on the gastrointestinal tract by inducing the secretion of prostaglandin E2 (PGE2) (Manneck et al. 2021). In addition to the above, TRPA1 is activated by cold, heat, and mechanical stimuli (Talavera et al. 2020). Caffeine can activate mouse TRPA1 channels, but suppresses human TRPA (Nagatomo and Kubo 2008). Many traditional herbal medicines might exert their pharmacological activity through modulating the activity of TRP channels (Sanechika et al. 2021), including petasites or parthenolide, safranal might exert analgesic properties by partial agonism and selective desensitization of the TRPA1 channel (Li Puma et al. 2019). The other herbal extracts, flavonoid aglycones, and glycycoumarin activated TRPA1 (Sanechika et al. 2021).
TRPA1 Antagonists
TRPA1 antagonists are mainly divided into selective and nonselective antagonists; acyl-glucuronide metabolite of ibuprofen (De Logu et al. 2019), derivatives of dipyrone and pyrazolone (Nassini et al. 2015), A967079 (Chen et al. 2011), xanthine derivatives (such as HC-030031) (McNamara et al. 2007), and GDC-0334 are considered to be TRPA1 selective antagonists (Balestrini et al. 2021); gadolinium, amiloride, gentamycin, and ruthenium red are nonselective TRPA1 antagonists (Baraldi et al. 2010). Various antagonists have also been extensively studied in terms of diseases, Ibuprofen-acyl-glucuronide, for example, has been shown to reduce the early pain response to formalin by both local and systemic administration, this new effect may contribute to the analgesic and anti-inflammatory activity of maternal drugs (De Logu et al. 2019). Such as the treatment of allergic rhinitis with HC-030031 (Fang et al. 2021), which has also been observed to help modulate depression- and anxiety-related behaviors in mice (de Moura et al. 2014). It was also found in animal experiments that GDC-0334 inhibited allergen-induced pulmonary neurogenic inflammation through the regulation of SP (Balestrini et al. 2021).
Both agonists or antagonist of TRPA1 might exert protective or harmful effects through different pathways. How to maximize its protective effect and reduce damage is an issue worthy of our in-depth study, research, and discussion.
TRPA1 and Disease
Nervous System
Migraine
Migraine mainly manifests as headache, nausea, vomiting, and hypersensitivity to stimuli such as light and sound. In some cases, there are also precursors (Benemei and Dussor 2019). Migraine aura is currently thought to be associated with cortical spreading depression (CSD), while migraine is associated with activation of the trigeminal neurovascular system (Goadsby and Holland 2019; May and Schulte 2016). After CSD, oxidative stress spreads downstream within the trigeminal nociceptive system and may be involved in the coupling of CSD to trigeminal vasculature activation in migraine pathology (Shatillo et al. 2013). A pathophysiological link between migraine and meningeal trigeminal innervation was proposed as early as 1979 (Kleeberg-Hartmann et al. 2021). Trigeminal nociceptors that innervate the cranial dura are sensitive to chemical and mechanical stimuli, suggesting that activation of these fibers may trigger headaches (Edelmayer et al. 2012).
TRPA1 receptor channels are localized to subpopulations of unmyelinated or thinly myelinated C- or Aδ fiber neurons in the dorsal root ganglia, trigeminal ganglia, and vagal ganglia and are almost exclusively expressed by C fibers present in the same nociceptive neuron (Benemei et al. 2014). Numerous studies have shown that meningeal TRPA1 may mediate migraine responses to environmental stimuli, one of the most common triggers of migraine (Edelmayer et al. 2012). For example, hydrogen sulfide (H2S) and nitric oxide (NO) can cause headaches (Benemei and Dussor 2019) and are activated in three ways: (1) cysteine residues of TRPA1 channels are targets of NO, and NO nitrosylation may contribute to channel sensitization (Demartini et al. 2017); (2) when H2S and NO combine to form nitroxyl, the two compounds work together to react with and activate TRPA1 through covalent modification (Benemei and Dussor 2019); and (3) polysulfides (H2Sn) generated from the interaction between H2S and NO also activate TRPA1 (Talavera et al. 2020).
How does the activation of TRPA1 mediate pain perception? Nakamura et al. proposed that TRPA1 activation by phosphorylating p38 mitogen-activated protein kinase releases SP from primary sensory neurons by increasing intracellular Ca2+ (an inflammatory response) while also inducing CGRP release from trigeminal neurons and dura mater tissue. In addition, TRPA1 may activate oxidative stress by inducing the occurrence of intracellular Ca2+ overload, resulting in increased release of intracellular inflammatory factors, neuroinflammation, and migraine (Demartini et al. 2017). CGRP can act in both the periphery to enhance nociceptor sensitization and the CNS to enhance sensory input, thereby heightening pain perception (Russo 2015).
TRPA1 is sensitive to oxidative stress and is also the target of emerging drugs involved in migraine prevention. The data show that the critical role of TRPA1 in regulating cortical susceptibility to CSD is functionally related to ROS and CGRP and that this role is the central mechanism. Because ROS trigger TRPA1 activation and CGRP production, they create a positive feedback loop in regulating cortical susceptibility to CSD. Through this pathway, ROS promote CSD dissemination for the subsequent development of migraine. Therefore, ROS/TRPA1/CGRP signaling contributes to CSD induction (Jiang et al. 2019) (Fig. 3). Efficacy of monoclonal antibodies against CGRP or its receptor (calcitonin receptor-like receptor/receptor activity modifying protein-1, CLR/RAMP1) implicates peripherally released CGRP in migraine pain. CLR/RAMP1 activation in human and mouse Schwann cells generates long-lasting signals from endosomes that evoke cAMP-dependent formation of NO. NO, by gating Schwann cell TRPA1, releases ROS, which in a feed-forward manner sustain allodynia via nociceptor TRPA1 (De Logu et al. 2022).
TRPA1 affects various systems and various diseases by regulating Ca2+ concentrations and affecting downstream-related mechanisms. It affects migraine by promoting neuropeptide release through oxidative stress; affects AD by activating PP2B signaling; and affects COPD through specific mechanisms of EGFR, GSK3β, p38 MAPK, and β-catenin. Activation of the RAS-ERK/AKT/mTOR signaling pathway affects lung cancer. TRPA1 promotes upregulation of ABCA1 and cholesterol efflux involved in the development of atherosclerosis and protects necrotic myocardium through the CaN-NFAT-DYRK1A signaling pathway. Activation of Ca2+-dependent enzymes and downstream transcription factors promotes T-cell activation and reduces colonic inflammation. COPD: chronic obstructive pulmonary disease. AD, Alzheimer’s disease. ROS, Reactive oxygen species. SP, substance P. CGRP, calcitonin gene‐related peptide. IL-6, interleukin-6. IL-8, interleukin-8. PP2B, serine/threonine-protein phosphatase 2B. NF-κB, nuclear factor κB. NFAT, nuclear factor of activated T-cells. mTOR, mammalian target of rapamycin. GSK-3β, glycogen synthase kinase-3β. ERK, extracellular-signal-regulated kinase. AKT, protein kinase B. MAPK, Mitogen-activated protein kinase. MU5AC, mucins 5AC. CaM, calmodulin. Pyk2, Proline-rich tyrosine kinase 2. Ras, rat sarcoma. ABCA1, ATP-binding cassette transporter A1. CaN, calcineurin. DYRK1A, dual-specificity tyrosine-regulated kinase-1a. NADPH, Nicotinamide adenine dinucleotide phosphate
About “Headaches” another neurological disease that affects us in the Lenovo—Progressive Multiple Sclerosis syndrome, but the mechanism is unclear. It is only known that an endogenous agonist of TRPA1 may sensitize TRPA1 in trigeminal nerve Nociceptor to trigger periorbital mechanical allodynia (Dalenogare et al. 2021).
Alzheimer’s Disease (AD)
AD is a type of neurodegenerative disease characterized by age-related cognitive and functional decline (Scheltens et al. 2021; Soria Lopez et al. 2019). Historically, AD was characterized by β-amyloid or Aβ from a pathological point of view (Dugger and Dickson 2017), but the current focus has expanded to include responses in other cell populations, such as microglia-mediated inflammation, which has taken center stage in functional studies of the pathogenesis of the disease (Scheltens et al. 2021).
In 2012, it was first reported that TRPA1 was expressed in astrocytes in the superficial layer of rat trigeminal caudal nucleus (Lee et al. 2012). Astrocytes are the most abundant cells in the central nervous system (CNS). These cells are able to transport ions, absorb neurotransmitters, and produce neurotrophic factors to maintain CNS function and homeostasis; among them, astrocyte ion channel plays a key role (Ikeshima-Kataoka 2016; Verkhratsky and Nedergaard 2018). Studies have shown that TRPA1 in astrocytes can be activated by ROS, NO, inflammatory factors, and pathological markers of neurodegenerative diseases such as Aβ, and it induces the inward flow of Ca2+ in astrocytes (Wang et al. 2022). Increased Ca2+ influx is a key event in the activation of serine/threonine-protein phosphatase 2B (PP2B) signaling and astrocyte inflammation (Lee et al. 2016). Imbalance of Ca2+ concentration leads to excessive activation of astrocytes, which in turn releases proinflammatory factors leading to neurodegeneration. Activation of nuclear factor (NF)-κB, PP2B, and nuclear factor NFAT, which activates T-cells, leads to an inflammatory response (Wang et al. 2022), thereby promoting the development of AD.
In the APP/PS1-21 mouse model of AD, blocking TRPA1 normalized astrocyte activity, avoided peri-synaptic astrocytic process regression, prevented neuronal dysfunction, and maintained structural synaptic integrity (Paumier et al. 2022). This indicates that the loss of TRPA1 channel function hinders the progression of AD. In summary, the activation and inhibition of TRPA1 channels have different effects on astrocytes. TRPA1-Ca2+-PP2B signaling may play a key role in regulating astrocyte-derived inflammation and AD pathogenesis (Lee et al. 2016). TRPA1 channels emerge as potential therapeutic targets for promoting neuroprotection.
Peripheral Neuropathy
Peripheral neuropathy here mainly includes chemotherapy-induced peripheral neuropathy (CIPN) and diabetic peripheral neuropathy (DPN). CIPN and its associated pain are a major side effect of some chemotherapy drugs used in cancer treatment (Moore et al. 2018). The chemotherapeutic drug oxaliplatin or one of its metabolites, oxalate, can inhibit the prolyl hydroxylase-mediated hydroxylation of an N-terminal proline residue of TRPA1, which induces TRPA1 sensitization to ROS and endows TRPA1 with cold sensitivity via transduction of ROS signaling (Nakagawa and Kaneko 2017). In a streptozotocin-induced diabetic mouse model, early cold hypersensitivity in DPN is mediated through TRPA1 sensitization during diabetic vascular injury (Hiyama et al. 2018). ROS play an important role in maintaining pain in neuropathic pain models. Selective ROS scavenging at the peripheral or central level, respectively, inhibits the corresponding TRPA1 components, thus inhibiting oxidative stress against TRPA1 contributes to the attenuation of mechanical allodynia (De Logu et al. 2020b). Schwann cell TRPA1 is required to coordinate neuroinflammation and oxidative stress to maintain neuropathic pain in complex areas of pain caused by ischemia–reperfusion (De Logu et al. 2020a). It has also been suggested that inhibition of TRPA1 activity by activation of AMPK, a ubiquitously expressed serine/threonine kinase, is a useful factor in the prevention of diabetic neuropathy (Wang et al. 2018). Overall, therapeutic strategies targeting TRPA1 for the treatment of painful peripheral neuropathy as well as various peripheral ischemic diseases such as peripheral arterial occlusive disease may be warranted (Hiyama et al. 2018).
Respiratory System
Asthma
Asthma is a respiratory disease characterized by airway inflammation, airflow obstruction, and airway hyperresponsiveness, mainly manifesting as cough, dyspnea, and wheezing (Balestrini et al. 2021). Although inflammation and the immune system play key roles in the pathogenesis of asthma, the efficacy of anti-inflammatory agents is limited, especially in patients with hormone-refractory asthma, suggesting that noncanonical pathways and cellular components are involved in disease manifestations.
TRPA1 is an ideal sensor for airway stimuli, is mainly expressed in small diameter nociceptive neurons, and is required for cigarette smoke (CS)-induced airway inflammation (Andrè et al. 2008). There are complex interactions between airway cells and nerve fibers. In humans, TRPA1 polymorphisms are associated with reduced asthma control (Balestrini et al. 2021). Based on the comprehensive review of relevant literature, the author believes that the effect of TRPA1 on asthma is mainly reflected in three aspects: participating in the inflammatory response, mediating the change of cough, and participating in the process of asthma exacerbation.
Caceres et al. recently showed that in a mouse model of allergic asthma, inhaled stimuli may activate TRPA1 expressed in sensory neurons innervating the airways, mediate inflammatory leukocyte infiltration and enhance pulmonary mucus production and airway hyperresponsiveness (Engel et al. 2011). The released neuropeptides induce bronchoconstriction, vasodilation, immune cell recruitment, and modulation of the inflammatory response. These effects promote protective physiological responses such as coughing, increased mucus secretion, and shallow breathing (Wu et al. 2021a). TRPA1 is an ion channel that mediates cough signaling (Luostarinen et al. 2021), which is key to the diagnosis and treatment of asthma. Cough is one of the important manifestations of asthma. The main types of fibers that cause cough are C fibers and Aδ fibers. The former has a slower conduction velocity, is unmyelinated, and is chemically sensitive, while the latter is myelinated, has fast conduction, and is more sensitive to mechanical stimuli (Bonvini and Belvisi 2017). Moreover, the airways have elevated levels of PGE2 and bradykinin (Choudry et al. 1989), both of which have been shown to activate TRPA1 (Grace et al. 2012) and cause cough (Choudry et al. 1989; Grace et al. 2014; Maher et al. 2009).
Th1/Th2 imbalance is the cause of allergic asthma (Li et al. 2019a). Allergic inflammation is driven by the activity of Th2 cytokines, which enhance TRP channels through multiple mechanisms under different inflammatory conditions (Meng et al. 2021). With the increase in asthma morbidity and mortality, asthma exacerbated by environmental pollution has attracted increasing attention. Trimellitic anhydride (TMA), a typical pollutant that is ubiquitous in the atmosphere, is a typical low-molecular-weight chemical sensitizer that can induce typical Th2 responses. OVA plus TMA-treated mice showed higher TRPA1 gene and protein expression and Th2 cytokine levels. Interestingly, similar results were detected in mice treated with OVA plus PM2.5 (Li et al. 2020a). This shows that environmental pollutants such as TMA can aggravate asthma (Li et al. 2019a), and this process may be related to the activation of the TRPA1 channel.
Chronic Obstructive Pulmonary Disease (COPD)
COPD is a persistent respiratory symptom caused by abnormalities of the airways and/or alveoli, such as coughing, wheezing, and airflow limitation, usually due to high exposure to toxic particles or gases (Soriano et al. 2018). TRPA1 has been identified as a pro-tussive receptor in both clinical trials and guinea pig models, and this effect can be blocked by selective antagonists (Belvisi et al. 2011). Therefore, TRPA1 not only has an impact on the pathogenesis of asthma but also may be related to the neurogenic mechanism of bronchitis and COPD in different stages. Both asthma and COPD cause airway obstruction and are associated with chronic inflammation of the airways. However, the nature and location of inflammation differ between the two, resulting in different pathologies, clinical manifestations, and responses to therapy (Barnes 2017). In summary, the effects of TRPA1 on the pathogenesis of COPD were mainly analyzed from the aspects of regulating airway mucus, bronchoconstriction, and inflammation.
Mucus hypersecretion is a pathological feature of acute inflammation and COPD (Memon et al. 2020). TRPA1 mediates CS-induced bronchial and alveolar epithelial cell injury by regulating airway mucus secretion, inflammation, smooth muscle contraction, and mitochondrial damage (Wang et al. 2019a). Studies have shown that exposure of primary human bronchial epithelial cells and mice to pine wood smoke particulate matter results in increased cytosolic calcium, increased phosphorylation of GSK3β, and increased nuclear entry after dephosphorylation of β-catenin owing to activation of TRPA1, thereby regulating the increased expression of mucin 5AC (MUC5AC), which eventually leads to an increase in mucus secretion (Memon et al. 2020). TRPA1 activation and intracellular Ca2+ can disrupt cellular integrity and cause EGFR ligand shedding and β-catenin accumulation in the cytoplasm, perinuclear region, and nucleus, in addition to being associated with the regulation of TRPA1 and EGFR downstream signaling. These effects occur through specific mechanisms involving TRPA1, EGFR, GSK3β, p38 MAPK, and β-catenin (Memon et al. 2020). Furthermore, TRPA1 channel-mediated Ca2+ release in endolysosomes directly triggers vesicular exocytosis and CGRP release, greatly enhancing DRG neuronal excitability. Thus, in addition to acting through Ca2+ influx, TRPA1 channels also trigger vesicle release in sensory neurons by releasing Ca2+ from lysosome-like organelles (Shang et al. 2016). Taken together, both epithelial and neuronal TRPA1 may be important in both nonallergic and allergic mechanisms leading to abnormal mucus hypersecretion in humans.
In recent years, nonneuronal localization of functional TRPA1 receptors has been demonstrated in muscle cells of airway smooth muscle (ASM) bundles (Wang et al. 2019b). ASM contraction controls airway caliber and can coordinate airway inflammation and remodeling (Jha et al. 2015). CS or extract exposure rapidly activates Ca2+ influx through TRPA1 into human airway smooth muscle cells, resulting in phosphorylation of myosin light chains and regulation of ASM contractility (Spix et al. 2022). The TRPA1 channel may be a determinant of ASM contraction, part of a novel mechanism that controls (pathological) airway, and ASM physiology (Jha et al. 2015).
It is well known that TRPA1 may be a key gatekeeper in regulating inflammatory responses to stimuli, including bacterial endotoxins, environmental irritants or inflammatory mediators (Lee et al. 2016). The researchers used the TRPA1-specific antagonist HC-030031 to abolish neuropeptide-mediated constriction of isolated guinea pig bronchial segments after perfusion with α, β-unsaturated aldehydes or CSE; in contrast, in TRPA1-deficient mice, instillation of CSE into the trachea failed to trigger neurogenic plasma extravasation. This suggests that the interaction of electrophilic components in CS with TRPA1 is the main mechanism by which CS induces acute neurogenic inflammation of the airways (Bessac and Jordt 2008).
Lung Cancer
There are also many established links between cancer and ion channels, and TRP channels are also oncogenic intracellular ion channels (Grimm et al. 2018). Among all TRP channels, TRPA1 was the most highly upregulated in lung squamous cell carcinoma and was the second most highly upregulated in lung adenocarcinoma (LUAD) (Takahashi et al. 2018). It has been shown that using agonists and antagonists of TRPA1 channel, respectively, can significantly increase and decrease Ca2+ levels in cancer cells, thus affecting the initiation and development of apoptosis (Özkal and Övey İ, 2020).
TRPA1 increases the phosphorylation of proline-rich tyrosine kinase 2 (PYK2), can be elevated by [Ca2+] via calmodulin (CaM) and can activate the RAS-ERK/AKT/mTOR signaling pathway (Cullen and Lockyer 2002), which mediates Ca2+ influx through Ca2+-CaM-mediated oxidative conditions. PYK2 activation amplifies the prosurvival/antiapoptotic signaling pathway (Takahashi et al. 2018). TRPA1 has also been shown to accelerate metabolism in cancer cells that require high concentrations of reactive oxygen species to maintain their high proliferation rates (Sosa et al. 2013). Oxidative stress defense is particularly important for cancer cells to gain anchorage independence, and TRPA1 channels can be directly activated by oxidants/electrophiles through cysteine modification and have the highest oxidative sensitivity (De Logu et al. 2021b; Takahashi et al. 2018). TRPA1 induces Ca2+ influx in response to ROS generated in the inner cells of tumor spheroids and protects them from apoptotic death (Takahashi et al. 2018).
With regard to LUAD, it has been demonstrated that the membrane receptor fibroblast growth factor receptor 2 (FGFR2) is a key driver of disease progression. FGFR2 recruits proteins to a proline-rich motif at the C-terminus of TRPA1, resulting in receptor phosphorylation and subsequent activation of downstream signaling pathways that promote LUAD progression and showing that astrocytes antagonize brain metastases by mediating the downregulation of TRPA1 via exosome-delivered miRNA-142-3p (Berrout et al. 2017). Furthermore, it has been found that elimination of Schwann cell TRPA1 attenuates lung cancer-induced mechanical allodynia, and therefore, Schwann cell TRPA1 may represent a potential target for the treatment of cancer pain (De Logu et al. 2021a).
Circulatory System
Atherosclerosis
Atherosclerosis, characterized by arterial wall hardening and arterial lumen narrowing, is thought to be a ROS-induced low-density lipoprotein (LDL) chronic inflammatory disease caused by oxidation (Wang et al. 2019b; Zhao et al. 2016). Ion channels play a key role in vascular disease (Wang et al. 2020); among them, the activation of the TRPA1 channel has a protective effect on the development of atherosclerosis (Wang et al. 2019b), and its dependent antiatherosclerotic effect may require the cooperation of multiple physiological pathways: regulating the phenotypic plasticity of macrophages, mediating ROS, and participating in vascular inflammation in the pathophysiological process of atherosclerosis (Moriya 2019), while macrophages are an important component in inflammatory infiltration and are present in all stages of the disease (Bartlett et al. 2019).
Macrophages are classified into the M1 and M2 types; M2 macrophages are atheroprotective and show anti-inflammatory effects, while M1 macrophages are largely involved in the expansion and progression of atherosclerotic lesions (Bartlett et al. 2019). Qiang Wang et al. demonstrated by RNA-seq that TRPA1 can regulate H3K27me3 (closed chromatin whose modification is regulated by PRC2) by protecting EZH2 (histone methyltransferase-active protein, one of the subunits of PRC2) from degradation regulation) to inhibit macrophage activation. TRPA1 deficiency leads to EZH2 degradation and chromatin opening, promoting M1 macrophage-associated gene transcription and atherosclerotic plaque formation (Wang et al. 2020). In response to atherosclerotic stimulation, TRPA1 is activated, and vascular cells release ROS, which stimulate smooth muscle cell migration and collagen deposition, leading to the development of atherosclerotic plaques (Kattoor et al. 2017).
The initial event in the development of atherosclerosis is endothelial injury. This causes infiltration into and accumulation of LDL cholesterol in the subendothelial space (Kattoor et al. 2017). LDL becomes oxidized to form oxidized LDL (ox-LDL) in pathologic states (Ketelhuth and Hansson 2011).The expression of TRPA1 in atherosclerotic lesions mainly occurs in the foam cell area of macrophages, and the most important atherosclerotic molecule, ox-LDL, increases the intracellular Ca2+ level through TRPA1 in macrophages and promotes the upregulation of ATP-binding cassette transporter A1 (ABCA1) and cholesterol efflux, suggesting that macrophage TRPA1 may be involved in atherogenic molecule-induced dysregulation of cholesterol metabolism and inflammation and the development of atherosclerosis through its influx of Ca2+ (Zhao et al. 2016).
Myocardial Infarction (MI)
MI is defined as sudden ischemic death of myocardial tissue. Clinically, MI is usually a thrombotic occlusion of a coronary vessel due to rupture of a vulnerable plaque. Ischemia causes severe metabolic and ionic perturbations in the affected myocardium and results in a rapid depression of contractile function (Frangogiannis 2015). TRPA1 is located in the sarcolemma and intercalated discs in cardiomyocytes (Üstünel and Özgüler 2021) and contributes to acrolein-induced calcium overload and hypercontraction (Conklin et al. 2019). Inhibition of TRPA1 promotes angiogenesis after MI, thereby attenuating myocardial ischemic injury through a mechanism of inhibition of phosphatase and tensin homolog expression and subsequent activation of PI3K/Akt signaling (Li et al. 2020b), suggesting that ischemia‒reperfusion (I/R) activation of TRPA1 exacerbates MI; this channel may be a potential target for alleviating I/R injury (Conklin et al. 2019).
After MI, physiological compensatory mechanisms promote cardiomyocyte loss and fibrosis through pathological remodeling. CF is a key cellular component of left ventricular remodeling after MI and is a major contributor to cardiac fibrosis. After MI stimulation, CFs are activated to proliferate, differentiate into cardiac myofibroblasts (CMFs), and play an important role in the fibrotic healing response (Hao et al. 2019; Li et al. 2019b). Methylglyoxal (MG, a highly active dicarbonyl compound) activates TRPA1 with deleterious cardiovascular effects associated with the activation of fibrosis. Inhibition of TRPA1 can reduce MG-induced Ca2+ influx, inhibit MG-induced fibroblast proliferation, and increase α-smooth muscle actin expression (Wang et al. 2019b). In an in vitro study, surprisingly, TRPA1 overexpression fully activated CMF transformation, while CF lacking TRPA1 induced transdifferentiation to transforming growth factor β- (TGF-β-), which promotes the Ca2+-responsive activation of calcineurin (CaN). Furthermore, dual-specificity tyrosine-regulated kinase 1a (DYRK1A) regulates CaN-mediated nuclear translocation of NFAT and TRPA1-dependent transdifferentiation. In summary, TRPA1 promotes the differentiation of CMFs after myocardial infarction injury through the CaN-NFAT-DYRK1A signaling pathway, thereby exerting a protective effect on the heart (Li et al. 2019b).
Digestive System
Appetite, Taste
Taste, which includes sweet, bitter, umami, salty, and sour, induces changes in Ca2+ levels, pH, and/or membrane potential in taste cells of the tongue and/or neurons that transmit and decode taste signals to the brain (von Molitor et al. 2020). These taste qualities are detected by distinct subsets of cells in the taste buds. Three distinct taste receptor cell subsets within taste buds are characterized as type I to type III cells. Type III cells express the TRP cation channel and the acid-sensing H+ channel otopetrin-1, among others (Rhyu et al. 2021). In many cases, TRP channels function directly as receptors for certain chemosensory stimuli (Aroke et al. 2020). TRPA1 is the Drosophila melanogaster ortholog of the human stimulatory sensor (Kang et al. 2010) and represents the first TRP required in separate chemosensory and thermosensory receptor cells, which function in taste and temperature discrimination (Kim et al. 2010). Drosophila responses to higher levels of bitter compounds are mediated by direct activation of TRPA1 (Leung et al. 2020), and deletion of TRPA1 results in reduced oral sensory sensitivity to menthol in mice (Lemon et al. 2019).
TRPA1 is also a structurally related thermosensitive cation channel, and the coexpression of this calcium-conducting TRP channel with CGRP in oral trigeminal C and Aδ fibers has been clearly reported (Kichko et al. 2018). TRPA1 is expressed in taste receptor neurons that respond to aversive compounds (Kim et al. 2010) and can be indirectly affected by the release of substance P and CGRP from trigeminal neurons; their subsequent effects on CGRP receptors expressed in type III taste receptor cells affect some but not all primary taste qualities (Rhyu et al. 2021).
Kazuaki Ohara et al. found that TRPA1 channels are involved in β-eudesmol (an oxygenated sesquiterpene present in medicinal or edible plants) regulation of feeding behavior, since there is a TRPA1-derived gastric vagal nerve activity (Ohara et al. 2017). In addition, single nucleotide polymorphisms in TRPA1 have been shown to affect and modulate chemosensation and taste.
Pancreatic Cancer
Pancreatic cancer is one of the leading causes of cancer-related deaths worldwide. Despite advances in early detection and treatment, the prognosis remains dismal (Manrai et al. 2021). Aberrant expression and/or activity of ion channels may lead to malignant transformation and tumor progression. TRP channels have been shown to play a role in Pancreatic cancer biology (Shi et al. 2022), TRPA1 is expressed in the pancreatic ductal adenocarcinoma cell (PDAC) cell lines Panc-1, MIA Paca-2, and BxPC-3 (Cojocaru et al. 2021), and it is located at the interface between the intracellular and extracellular spaces in the cell membrane, sensing and modifying the tumor microenvironment; this in itself is a driver of the aggressiveness of PDAC (Hofschröer et al. 2020).
Relevant information on the impact of TRPA1 on Pancreatic cancer is relatively limited. According to the existing reports, the summary is as follows.
First, researchers have identified that silencing TRPA1 expression induces a significant increase in migration potential and that TRPA1 channels use pore-independent signaling pathways to participate in migration (Cojocaru et al. 2021).
Second, nonselective cation currents were activated by AITC in Panc-1 cells and inhibited by the selective TRPA1 antagonist A-967079 (Cojocaru et al. 2021), indicating that the cation current is regulated by TRPA1. It is well known that calcium signaling drives key oncogenic processes such as proliferation, migration, invasion, and angiogenesis (Kutschat et al. 2021). During proliferation, Ca2+ has a fundamental role in cell cycle initiation and progression, as evidenced by the inhibition of several Ca2+ channels leading to cell cycle arrest (Mesquita et al. 2021). Based on the above information, we infer that TRPA1 may affect the cell cycle of PDAC through the regulation of Ca2+, but this possibility remains to be further studied and explored.
Finally, cancer cell survival depends on oxidative stress defense against ROS that accumulate during tumorigenesis. Although there is currently no evidence that TRPA1 is directly involved in the process of Pancreatic cancer oxidative stress, there are data showing that NRF2, an oxidant defense transcription factor, directly controls the expression of TRPA1; more notably, the KEAP1-NRF2 pathway, which plays a central role in protecting cells from oxidative stress by inducing ROS-neutralizing gene expression, was shown to stimulate carcinogenesis and support tumor maintenance in Pancreatic cancer (Takahashi et al. 2018). Taken together, these findings indicate that TRPA1 channels in PDAC cell membranes regulate cellular processes through pore-dependent and pore-independent mechanisms (Cojocaru et al. 2021). The putative role of endogenous channels and activators opens new perspectives to study TRPA1 in PDAC and nontumor cell lines, and TRPA1 channels are promising as PDAC antitumor therapy (Cojocaru et al. 2021).
Inflammatory Bowel Disease
Inflammatory Bowel Disease refers to ulcerative colitis (UC) and Crohn’s disease (CD) and is characterized by chronic idiopathic inflammation (Sairenji et al. 2017). In addition to genetic susceptibility, there is also a certain relationship with the immune response (Zhang and Li 2014). TRPA1 has a broad tissue distribution, and TRPA1 mRNA and protein were also detected in intestinal epithelial cells (Bertin et al. 2017). TRPA1 is also reported to be upregulated in Inflammatory Bowel Disease patients and plays a crucial role in the inflammatory response of Inflammatory Bowel Disease (Wu et al. 2021a).
As a mechanosensor, activation of TRPA1 is thought to be a factor in the generation of afferent mechanical hypersensitivity in models of colitis. TRPA1 is also activated by the release of inflammatory mediators when tissue is damaged or diseased (Hassan et al. 2020). TRPs essentially control the vesicular exocytosis of CGRP by virtue of their inherent Ca2+ conductance, nicotinic acetylcholine receptor channels depolarize nerves, and the support of voltage-gated calcium channels is then required to trigger CGRP release (Kichko et al. 2018). Meanwhile, upregulation of TRPA1 at the level of primary sensory neurons also contributes to the release of SP and CGRP. Inhibition of TRPA1 thus reduces the inflammatory process by reducing colonic neuropeptide (substance P and CGRP) release from sensory neurons outside the gut. In addition, TRPA1 was also detected in CD4+ T-cells infiltrating colon tissue samples from patients with UC and CD (Landini et al. 2022), and TRPA1 is involved in the control of CD4+ T-cell activation and proinflammatory responses in two different T-cell-mediated colitis models (Bertin et al. 2017). Among immune cells in the gut, T-cells play an important role in maintaining gut immunity and homeostasis (Wu et al. 2021c), and immune cells are heavily dependent on the Ca2+ signaling pathway. For example, antigen recognition by T-cell receptors results in IP3-dependent release of Ca2+ from the endoplasmic reticulum. This reduction in endoplasmic reticulum Ca2+ levels results in the activation of Ca2+ release-activated Ca2+ channels in the membrane and the influx of extracellular Ca2+. This in turn activates Ca2+-dependent enzymes and downstream transcription factors, such as NF-κB and NFAT, which then lead to T-cell activation (Naert et al. 2021). Thus, TRPA1 in CD4+ T-cells appears to reduce the severity of T-cell-mediated colitis.
Intestinal fibrosis is a common complication of Inflammatory Bowel Disease, affecting 30%–50% of CD patients. It is characterized by the accumulation of myofibroblasts and excessive deposition of extracellular matrix (Hirota 2018). However, in some clinical studies, elevated levels of TGF-β1 mRNA were found in the intestinal mucosa of CD and UC patients, especially in the lamina propria region of immune cells and myofibroblasts. Combining this fact with our own findings in human surgical samples (i.e., coaccumulation of TRPA1-/HSP47- double-positive myofibroblasts in stenotic areas) (Hiraishi et al. 2018), we speculate that TRPA1 not only has a protective effect on the intestine but also has a certain effect on intestinal fibrosis caused by intestinal by targeting the TRPA1 signaling axis of myofibroblasts.
Urinary System
Acute Kidney Injury
The causes of Acute kidney injury are traditionally divided into three categories: prerenal, renal (with direct intrinsic renal injury), and postrenal. Approximately, two-thirds of acute tubular necrosis is caused by renal ischemia‒reperfusion injury (IRI) or sepsis, and one-third is caused by direct or indirect nephrotoxicity (Lameire et al. 2013). However, the author believes that oxidative stress and inflammation play an important role in the pathophysiology of renal ischemia‒reperfusion or other causes of nephrotoxicity.
TRPA1 expression was significantly increased in renal tubular epithelial cells both in patients with Acute kidney injury and in an in vitro model (under hypoxia-reoxygenation (H/R) conditions). During the reperfusion phase of IR, excess ROS are generated, leading to increased oxidative stress in renal tissue. In addition to being sensitive to ROS, TRPA1 is also highly permeable to Ca2+. H/R induced ROS-dependent TRPA1 activation, which increased intracellular Ca2+ levels, increased NADPH oxidase activity, activated MAPK/NF-κB signaling, and promoted the release of the inflammatory factor IL-8. Therefore, tubular TRPA1 is a sensor of oxidative stress and a key regulator of activated signaling pathways (Wu et al. 2021b). Similar to IRI, ROS also activate MAPK and NF-κB signaling in cisplatin (DDP, a commonly used chemotherapeutic drug)-induced nephrotoxicity. More importantly, treatment of HEK293 cells with the TRPA1 antagonist HC-030031 reduced the expression of phosphorylated IκBα, IKKβ, JNK, ERK, and p38. TRPA1 regulates the phosphorylation of the MAPK/NF-κB signaling pathway, promotes the production and release of inflammatory cytokines and mediators, and mediates DDP-induced cellular inflammation and apoptosis through the MAPK/NF-κB signaling pathway (Yuan et al. 2021).
In addition, TRPA1 in renal tubular epithelial cells was identified to be expressed in macrophages, and the role of macrophages in IRI cannot be underestimated. The TRPA1 activator AITC was able to attenuate macrophage activation and foam cell formation (Ma et al. 2019); the same pathway mediates H/R injury in vitro and prevents Ang-II-induced renal injury and ischemia‒reperfusion renal injury in vivo by maintaining mitochondrial hemostasis and downregulating macrophage-mediated inflammatory responses (Ma et al. 2019; Wu et al. 2021a).
Lower Urinary Tract Dysfunction
Lower Urinary Tract dysfunction is a common sequelae of neurological disorders. While it is mostly not fatal, the associated social disturbances, especially reduced quality of life, should not be underestimated (Franken et al. 2014; Panicker et al. 2015). In the past 20 years, TRP channels have become increasingly important in this field of research (Franken et al. 2014), with important sensory functions in lower urinary tract symptoms (LUTs) (Deruyver et al. 2015), because TRPA1 activation triggers pain have been demonstrated in human urothelial cells and C-fiber afferents in the lamina propria and detrusor muscle, and a role for TRPA1 in afferent and efferent sensory signaling in human outflow regions has been suggested. Intravesical TRPA1 activators can initiate detrusor overactivity, pain perception (suprapubic pain, dysuria…), and/or heat sensitivity (“burning” sensation, bladder cooling reflex). Alternatively, these proteins can be targeted to alter sensory nerve function (excitability), and a role in sensory transduction in LUTs has been supported by animal experiments (Andersson 2019; Deruyver et al. 2015).
The possibility that the bladder is innervated by at least two afferent nerves has been proposed: type A expresses the TRPA1 receptor, which induces PGE release and excites the detrusor, and type B expresses the TRPV1, TRPA1, and TRPC receptors and releases CGRP that inhibits the detrusor (Daugherty et al. 2021). Increased expression of TRPA1 in the bladder wall is associated with the establishment of overactive bladder and lower urinary tract symptoms, TRPA1 activation triggers pain, protective reflexes, and local release of peripheral neurotransmitters and is associated with spontaneous and involuntary bladder contractions in spinal cord injury (Blaha et al. 2019; Wu et al. 2021a). The effect of its activation on bladder contractility has been attributed to stimulation of TRPA1-expressing sensory nerve fibers causing them to release SP and PGE2, each capable of activating contractile cells through tachykinin and PGE receptors on the surface of detrusor smooth muscle (Philyppov et al. 2016). Studies have also shown that HC-030031 treatment reduces the number and magnitude of nonvoiding contractions (NVCs), and inhibition of TRPA1 can effectively reduce bladder activity; TRPA1 antisense oligonucleotide treatment normalized spontaneous phase activity and reduced CA-induced bladder contractions and NVC numbers in spinal cord injury rats (Andrade et al. 2011).
Others
Retinal Damage
I/R damage underlies many retinal diseases, such as glaucoma, diabetic retinopathy, and central retinal artery occlusion, a leading cause of visual impairment or blindness (Wan et al. 2020). Low-level ROS production, mainly by mitochondria, is necessary for the maintenance of physiological functions; however, ROS can also be dangerous. For example, oxidative stress caused by excess reactive oxygen species can lead to retinal ganglion cell death (McMonnies 2018). When blood supply is re-established after prolonged ischemia, local inflammation and production of reactive oxygen species increase, leading to secondary injury (Wu et al. 2018). Studies have found that human retinal cells express TRPA1 (mRNA and protein). Genetic deletion or pharmacological blockade of TRPA1 attenuated I/R-induced increases in infiltrating macrophage numbers and levels of the oxidative stress biomarker 4-hydroxynonenal and the apoptosis biomarker active caspase-3. These findings suggest that TRPA1 mediates oxidative stress load and inflammation that lead to retinal cell death in mice; inhibition of TRPA1-dependent pathways may also alleviate glaucoma-related retinal damage (Souza Monteiro de Araúja et al. 2020a).
Melanoma
Melanoma is a type of skin cancer caused by malignant tumors of melanocytes (Ahmed et al. 2020). TRPA1 has been found in melanocytes and keratinocytes (Chen and Hackos 2015). On this basis, it was also found that treatment of keratinocytes with the selective TRPA1 agonist icilin increased the expression of genes involved in cell adhesion and extracellular matrix protein synthesis (Maglie et al. 2021). During melanoma formation, macrophages, especially tumor-associated macrophages and ROS, are involved in all stages of melanogenesis (Chen et al. 2019; De Logu et al. 2021b). ROS released by infiltrating M2 macrophages may target TRPA1-expressing melanoma cells to amplify oxidative stress signals that affect tumor cell survival and proliferation. It can therefore be said that TRPA1 acts as an oxidative stress sensor and amplifier, contributing to cancer progression and metastasis (De Logu et al. 2021b). Second, TRPA1 drug blockade also reduced dacarbazine-induced nociception in a melanoma tumor-associated pain model, suggesting that this receptor may be a pharmacological agent for chemotherapy-induced pain syndrome in cancer patients receiving dacarbazine antitumor therapy (Brusco et al. 2020).
Diabetes
Diabetes mellitus is a series of metabolic disorder syndromes, including protein, fat, and electrolytes, caused by the absolute or relative insufficient secretion of insulin and the decreased sensitivity of target tissue cells to insulin, with hyperglycemia as the main sign. TRP channels play an important role in mediating glucose-stimulated insulin release by causing depolarization of pancreatic β cells and closure of KATP channels (Adhya and Sharma 2019).
TRPA1 stimulates insulin secretion in diabetic beta cells and improves hyperglycemia. It has been reported that dual agonists of the TRPA1/GPR-119 receptor in intestinal STC-1 cells induce the cells to secrete glucagon-like peptide-1 (GLP-1). The released GLP-1 then causes the secretion of insulin release by acting on the GLP-1 receptor in beta cells (Bae and Sun 2011). In fact, TRPA1 agonists themselves can also activate mouse enterocytes to release GLP-1 (Adhya and Sharma 2019). Furthermore, in mouse beta cells and INS-1 cells, catechol estrogens activate TRPA1 channels, increase [Ca2+]i, and stimulate insulin secretion in a glucose-dependent manner. These effects were inhibited by pharmacological inhibitors of TRPA1 and siRNA (Islam 2020). In addition, the antidiabetic drug glyburide has also been reported to activate the TRPA1 channel, which may explain its antidiabetic effect as well as its ability to block KATP channels (Adhya and Sharma 2019).
Conclusions
From our exposition of the pathogenesis of TRPA1 involved in various diseases, it can be seen that the current research on the effect of this channel on some diseases is still in its infancy, and the research on the role of TRPA1 channel is still complex and arduous, but we have also some gains. It was found that the effects of some different diseases also have some commonalities. The participation of the TRPA1 channel is a key mechanism for the occurrence and development of certain diseases. These include the release of CGRP and SP in pain (headache), inflammation (colitis), and appetite regulation. For example, the regulation of oxidative stress in the pathogenesis of cancer (lung cancer, pancreatic cancer), blood vessels (atherosclerosis), and ischemia‒reperfusion (kidney injury) are mostly achieved through the regulation of Ca2+. In conclusion, TRPA1 has emerged as a central therapeutic target for the treatment of multiple pathologies with a common etiology, offering an attractive therapeutic possibility for multiple diseases.
References
Adhya P, Sharma SS (2019) Redox TRPs in diabetes and diabetic complications: mechanisms and pharmacological modulation. Pharmacol Res 146:104271
Ahmed B, Qadir MI, Ghafoor S (2020) Malignant melanoma: skin cancer-diagnosis, prevention, and treatment. Crit Rev Eukaryot Gene Expr 30:291–297
Andersson KE (2019) Agents in early development for treatment of bladder dysfunction—promise of drugs acting at TRP channels? Expert Opin Investig Drugs 28:749–755
Andrade EL, Forner S, Bento AF, Leite DF, Dias MA, Leal PC, Koepp J, Calixto JB (2011) TRPA1 receptor modulation attenuates bladder overactivity induced by spinal cord injury. Am J Physiol Renal Physiol 300:F1223-1234
Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M et al (2008) Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118:2574–2582
Aroke EN, Powell-Roach KL, Jaime-Lara RB, Tesfaye M, Roy A, Jackson P, Joseph PV (2020) Taste the pain: the role of TRP channels in pain and taste perception. Int J Mol Sci 21:5929
Bae CY, Sun HS (2011) TRPM7 in cerebral ischemia and potential target for drug development in stroke. Acta Pharmacol Sin 32:725–733
Balestrini A, Joseph V, Dourado M, Reese RM, Shields SD, Rougé L, Bravo DD, Chernov-Rogan T, Austin CD, Chen H et al (2021) A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J Exp Med. https://doi.org/10.1084/jem.20201637
Baraldi PG, Preti D, Materazzi S, Geppetti P (2010) Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J Med Chem 53:5085–5107
Barnes PJ (2017) Cellular and molecular mechanisms of asthma and COPD. Clin Sci (London: 1979) 131:1541–1558
Bartlett B, Ludewick HP, Misra A, Lee S, Dwivedi G (2019) Macrophages and T cells in atherosclerosis: a translational perspective. Am J Physiol Heart Circ Physiol 317:H375-h386
Belvisi MG, Dubuis E, Birrell MA (2011) Transient receptor potential A1 channels: insights into cough and airway inflammatory disease. Chest 140:1040–1047
Benemei S, Dussor G (2019) TRP channels and migraine: recent developments and new therapeutic opportunities. Pharmaceuticals (Basel) 12:54
Benemei S, Fusi C, Trevisan G, Geppetti P (2014) The TRPA1 channel in migraine mechanism and treatment. Br J Pharmacol 171:2552–2567
Berrout J, Kyriakopoulou E, Moparthi L, Hogea AS, Berrout L, Ivan C, Lorger M, Boyle J, Peers C, Muench S et al (2017) TRPA1-FGFR2 binding event is a regulatory oncogenic driver modulated by miRNA-142-3p. Nat Commun 8:947
Bertin S, Aoki-Nonaka Y, Lee J, de Jong PR, Kim P, Han T, Yu T, To K, Takahashi N, Boland BS et al (2017) The TRPA1 ion channel is expressed in CD4+ T cells and restrains T-cell-mediated colitis through inhibition of TRPV1. Gut 66:1584–1596
Bessac BF, Jordt SE (2008) Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23:360–370
Blaha I, López-Oliva ME, Martínez MP, Recio P, Agis-Torres Á, Martínez AC, Benedito S, García-Sacristán A, Prieto D, Fernandes VS et al (2019) Bladder dysfunction in an obese zucker rat: the role of TRPA1 channels, oxidative stress, and hydrogen sulfide. Oxid Med Cell Longev 2019:5641645
Bonvini SJ, Belvisi MG (2017) Cough and airway disease: the role of ion channels. Pulm Pharmacol Ther 47:21–28
Brusco I, Li Puma S, Chiepe KB, da Silva Brum E, de David Antoniazzi CT, de Almeida AS, Camponogara C, Silva CR, De Logu F, de Andrade VM et al (2020) Dacarbazine alone or associated with melanoma-bearing cancer pain model induces painful hypersensitivity by TRPA1 activation in mice. Int J Cancer 146:2797–2809
Chen J, Hackos DH (2015) TRPA1 as a drug target–promise and challenges. Naunyn Schmiedebergs Arch Pharmacol 388:451–463
Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, Segreti JA, Han P, Zhang XF, Niforatos W et al (2011) Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 152:1165–1172
Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z (2019) Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci 26:78
Choudry NB, Fuller RW, Pride NB (1989) Sensitivity of the human cough reflex: effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. Am Rev Respir Dis 140:137–141
Cojocaru F, Şelescu T, Domocoş D, Măruţescu L, Chiritoiu G, Chelaru NR, Dima S, Mihăilescu D, Babes A, Cucu D (2021) Functional expression of the transient receptor potential ankyrin type 1 channel in pancreatic adenocarcinoma cells. Sci Rep 11:2018
Conklin DJ, Guo Y, Nystoriak MA, Jagatheesan G, Obal D, Kilfoil PJ, Hoetker JD, Guo L, Bolli R, Bhatnagar A (2019) TRPA1 channel contributes to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 316:H889-h899
Cullen PJ, Lockyer PJ (2002) Integration of calcium and Ras signalling. Nat Rev Mol Cell Biol 3:339–348
Dalenogare DP, Ritter C, Bellinaso FRA, Kudsi SQ, Pereira GC, Fialho MFP, Lückemeyer DD, Antoniazzi CTD, Landini L, Ferreira J et al (2021) Periorbital nociception in a progressive multiple sclerosis mouse model is dependent on TRPA1 channel activation. Pharmaceuticals (Basel) 14:831
Daugherty SL, Beckel JM, Kim KA, Freeman BA, Liu J, Wang S, de Groat WC, Zhang X (2021) TRP channel agonists activate different afferent neuromodulatory mechanisms in Guinea Pig urinary bladder. Front Physiol 12:692719
De Logu F, Nassini R, Materazzi S, Carvalho Gonçalves M, Nosi D, Degl’Innocenti DR, Marone IM, Ferreira J, Li Puma S, Benemei S et al (2017) Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat Commun 8:1887
De Logu F, Li Puma S, Landini L, Tuccinardi T, Poli G, Preti D, De Siena G, Patacchini R, Tsagareli MG, Geppetti P et al (2019) The acyl-glucuronide metabolite of ibuprofen has analgesic and anti-inflammatory effects via the TRPA1 channel. Pharmacol Res 142:127–139
De Logu F, De Prá SD, de David Antoniazzi CT, Kudsi SQ, Ferro PR, Landini L, Rigo FK, de Bem Silveira G, Silveira PCL, Oliveira SM et al (2020a) Macrophages and Schwann cell TRPA1 mediate chronic allodynia in a mouse model of complex regional pain syndrome type I. Brain Behav Immun 88:535–546
De Logu F, Trevisan G, Marone IM, Coppi E, Padilha Dalenogare D, Titiz M, Marini M, Landini L, de Souza Monteiro Araujo D, Li Puma S et al (2020b) Oxidative stress mediates thalidomide-induced pain by targeting peripheral TRPA1 and central TRPV4. BMC Biol 18:197
De Logu F, Marini M, Landini L, de Souza Monteiro Araujo D, Bartalucci N, Trevisan G, Bruno G, Marangoni M, Schmidt BL, Bunnett NW et al (2021) Peripheral nerve resident macrophages and Schwann cells mediate cancer-induced pain. Cancer research 81:3387–3401
De Logu F, de Souza Monteiro Araujo D, Ugolini F, Iannone LF, Vannucchi M, Portelli F, Landini L, Titiz M, De Giorgi V, Geppetti P et al (2021) The TRPA1 Channel Amplifies the Oxidative Stress Signal in Melanoma. Cells 10:3131
De Logu F, Nassini R, Hegron A, Landini L, Jensen DD, Latorre R, Ding J, Marini M, de Souza Monteiro Araujo D, Ramírez-Garcia P et al (2022) Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat Commun 13:646
De Moura JC, Noroes MM, Rachetti Vde P, Soares BL, Preti D, Nassini R, Materazzi S, Marone IM, Minocci D, Geppetti P et al (2014) The blockade of transient receptor potential ankirin 1 (TRPA1) signalling mediates antidepressant- and anxiolytic-like actions in mice. Br J Pharmacol 171:4289–4299
de Souza Monteiro Araujo D, Nassini R, Geppetti P, De Logu F (2020) TRPA1 as a therapeutic target for nociceptive pain. Expert Opin Ther Targets 24:997–1008
de SouzaMonteiroAraujo D, De Logu F, Adembri C, Rizzo S, Janal MN, Landini L, Magi A, Mattei G, Cini N, Pandolfo P et al (2020) TRPA1 mediates damage of the retina induced by ischemia and reperfusion in mice. Cell death & disease 11:633
Demartini C, Tassorelli C, Zanaboni AM, Tonsi G, Francesconi O, Nativi C, Greco R (2017) The role of the transient receptor potential ankyrin type-1 (TRPA1) channel in migraine pain: evaluation in an animal model. J Headache Pain 18:94
Deruyver Y, Voets T, De Ridder D, Everaerts W (2015) Transient receptor potential channel modulators as pharmacological treatments for lower urinary tract symptoms (LUTS): myth or reality? BJU Int 115:686–697
Doerner JF, Gisselmann G, Hatt H, Wetzel CH (2007) Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem 282:13180–13189
Dugger BN, Dickson DW (2017) Pathology of neurodegenerative diseases. Cold Spring Harbor Persp Biol 9:a028035
Earley S (2012) TRPA1 channels in the vasculature. Br J Pharmacol 167:13–22
Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, Preti D, Appendino G, Geppetti P, Dodick DW et al (2012) Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain 153:1949–1958
Engel MA, Leffler A, Niedermirtl F, Babes A, Zimmermann K, Filipović MR, Izydorczyk I, Eberhardt M, Kichko TI, Mueller-Tribbensee SM et al (2011) TRPA1 and substance P mediate colitis in mice. Gastroenterology 141:1346–1358
Fang Z, Yi F, Peng Y, Zhang JJ, Zhang L, Deng Z, Chen F, Li C, He Y, Huang C et al (2021) Inhibition of TRPA1 reduces airway inflammation and hyperresponsiveness in mice with allergic rhinitis. FASEB J 35:e21428
Frangogiannis NG (2015) Pathophysiology of myocardial infarction. Compr Physiol 5:1841–1875
Franken J, Uvin P, De Ridder D, Voets T (2014) TRP channels in lower urinary tract dysfunction. Br J Pharmacol 171:2537–2551
Goadsby PJ, Holland PR (2019) An update: pathophysiology of migraine. Neurol Clin 37:651–671
Grace M, Birrell MA, Dubuis E, Maher SA, Belvisi MG (2012) Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax 67:891–900
Grace MS, Baxter M, Dubuis E, Birrell MA, Belvisi MG (2014) Transient receptor potential (TRP) channels in the airway: role in airway disease. Br J Pharmacol 171:2593–2607
Grimm C, Bartel K, Vollmar AM, Biel M (2018) Endolysosomal cation channels and cancer-a link with great potential. Pharmaceuticals (Basel) 11:4
Hao K, Lei W, Wu H, Wu J, Yang Z, Yan S, Lu XA, Li J, Xia X, Han X et al (2019) LncRNA-Safe contributes to cardiac fibrosis through Safe-Sfrp2-HuR complex in mouse myocardial infarction. Theranostics 9:7282–7297
Hassan AA, Sleet B, Cousins Z, Keating CD (2020) TRPA1 channel activation inhibits motor activity in the mouse colon. Front Neurosci 14:471
Hiraishi K, Kurahara LH, Sumiyoshi M, Hu YP, Koga K, Onitsuka M, Kojima D, Yue L, Takedatsu H, Jian YW et al (2018) Daikenchuto (Da-Jian-Zhong-Tang) ameliorates intestinal fibrosis by activating myofibroblast transient receptor potential ankyrin 1 channel. World J Gastroenterol 24:4036–4053
Hirota SA (2018) TRPing up fibrosis: a novel role for TRPA1 in intestinal myofibroblasts. Cell Mol Gastroenterol Hepatol 5:365
Hiyama H, Yano Y, So K, Imai S, Nagayasu K, Shirakawa H, Nakagawa T, Kaneko S (2018) TRPA1 sensitization during diabetic vascular impairment contributes to cold hypersensitivity in a mouse model of painful diabetic peripheral neuropathy. Mol Pain 14:1744806918789812
Hofschröer V, Najder K, Rugi M, Bouazzi R, Cozzolino M, Arcangeli A, Panyi G, Schwab A (2020) Ion channels orchestrate pancreatic ductal adenocarcinoma progression and therapy. Front Pharmacol 11:586599
Hu F, Song X, Long D (2021) Transient receptor potential ankyrin 1 and calcium: Interactions and association with disease (review). Exp Ther Med 22:1462
Ikeshima-Kataoka H (2016) Neuroimmunological implications of AQP4 in astrocytes. Int J Mol Sci 17:1306
Islam MS (2020) Molecular regulations and functions of the transient receptor potential channels of the islets of langerhans and insulinoma cells. Cells 9:685
Jha A, Sharma P, Anaparti V, Ryu MH, Halayko AJ (2015) A role for transient receptor potential ankyrin 1 cation channel (TRPA1) in airway hyper-responsiveness? Can J Physiol Pharmacol 93:171–176
Jiang L, Ma D, Grubb BD, Wang M (2019) ROS/TRPA1/CGRP signaling mediates cortical spreading depression. J Headache Pain 20:25
Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA (2010) Analysis of drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 464:597–600
Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL (2017) Oxidative stress in atherosclerosis. Curr Atheroscler Rep 19:42
Ketelhuth DF, Hansson GK (2011) Cellular immunity, low-density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thromb Haemost 106:779–786
Kichko TI, Neuhuber W, Kobal G, Reeh PW (2018) The roles of TRPV1, TRPA1 and TRPM8 channels in chemical and thermal sensitivity of the mouse oral mucosa. Eur J Neurosci 47:201–210
Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C (2010) Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci USA 107:8440–8445
Kleeberg-Hartmann J, Vogler B, Messlinger K (2021) Petasin and isopetasin reduce CGRP release from trigeminal afferents indicating an inhibitory effect on TRPA1 and TRPV1 receptor channels. J Headache Pain 22:23
Kutschat AP, Johnsen SA, Hamdan FH (2021) Store-operated calcium entry: shaping the transcriptional and epigenetic landscape in pancreatic cancer. Cells 10:966
Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W et al (2013) Acute kidney injury: an increasing global concern. Lancet (London) 382:170–179
Landini L, de Souza Monteiro Araujo D, Titiz M, Geppetti P, Nassini R, De Logu F (2022) TRPA1 role in inflammatory disorders: what is known so far? Int J Mol Sci 23:4529
Lee SM, Cho YS, Kim TH, Jin MU, Ahn DK, Noguchi K, Bae YC (2012) An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J Chem Neuroanat 45:45–49
Lee KI, Lee HT, Lin HC, Tsay HJ, Tsai FC, Shyue SK, Lee TS (2016) Role of transient receptor potential ankyrin 1 channels in Alzheimer’s disease. J Neuroinflammation 13:92
Lemon CH, Norris JE, Heldmann BA (2019) The TRPA1 ion channel contributes to sensory-guided avoidance of menthol in mice. eNeuro. https://doi.org/10.1523/ENEURO.0304-19.2019
Leung NY, Thakur DP, Gurav AS, Kim SH, Di Pizio A, Niv MY, Montell C (2020) Functions of opsins in drosophila taste. Curr Biol: CB 30:1367-1379.e1366
Li H (2017) TRP channel classification. Adv Exp Med Biol 976:1–8
Li M, Fan X, Ji L, Fan Y, Xu L (2019a) Exacerbating effects of trimellitic anhydride in ovalbumin-induced asthmatic mice and the gene and protein expressions of TRPA1, TRPV1, TRPV2 in lung tissue. Int Immunopharmacol 69:159–168
Li S, Sun X, Wu H, Yu P, Wang X, Jiang Z, Gao E, Chen J, Li D, Qiu C et al (2019b) TRPA1 promotes cardiac myofibroblast transdifferentiation after myocardial infarction injury via the calcineurin-NFAT-DYRK1A signaling pathway. Oxid Med Cell Longev 2019:6408352
Li M, Fan X, Yue Q, Hu F, Zhang Y, Zhu C (2020a) The neuro-immune interaction in airway inflammation through TRPA1 expression in CD4+ T cells of asthmatic mice. Int Immunopharmacol 86:106696
Li R, Liu R, Yan F, Zhuang X, Shi H, Gao X (2020b) Inhibition of TRPA1 promotes cardiac repair in mice after myocardial infarction. J Cardiovasc Pharmacol 75:240–249
Li Puma S, Landini L, Macedo SJ Jr, Seravalli V, Marone IM, Coppi E, Patacchini R, Geppetti P, Materazzi S, Nassini R et al (2019) TRPA1 mediates the antinociceptive properties of the constituent of Crocus sativus L., safranal. J Cell Mol Med 23:1976–1986
Luostarinen S, Hämäläinen M, Hatano N, Muraki K, Moilanen E (2021) The inflammatory regulation of TRPA1 expression in human A549 lung epithelial cells. Pulm Pharmacol Ther 70:102059
Ma S, Zhang Y, He K, Wang P, Wang DH (2019) Knockout of TRPA1 exacerbates angiotensin II-induced kidney injury. Am J Physiol Renal Physiol 317:F623-f631
Maglie R, de Souza Monteiro Araujo D, Antiga E, Geppetti P, Nassini R, De Logu F (2021) The role of TRPA1 in skin physiology and pathology. Int J Mol Sci 22:3065
Maher SA, Birrell MA, Belvisi MG (2009) Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. Am J Respir Crit Care Med 180:923–928
Manneck D, Manz G, Braun HS, Rosendahl J, Stumpff F (2021) The TRPA1 agonist cinnamaldehyde induces the secretion of HCO (3) (–) by the porcine colon. Int J Mol Sci 22:5198
Manrai M, Tilak T, Dawra S, Srivastava S, Singh A (2021) Current and emerging therapeutic strategies in pancreatic cancer: challenges and opportunities. World J Gastroenterol 27:6572–6589
May A, Schulte LH (2016) Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol 12:455–464
McMonnies C (2018) Reactive oxygen species, oxidative stress, glaucoma and hyperbaric oxygen therapy. J Optometry 11:3–9
McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM et al (2007) TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 104:13525–13530
Meents JE, Ciotu CI, Fischer MJM (2019) TRPA1: a molecular view. J Neurophysiol 121:427–443
Memon TA, Nguyen ND, Burrell KL, Scott AF, Almestica-Roberts M, Rapp E, Deering-Rice CE, Reilly CA (2020) Wood smoke particles stimulate muc5ac overproduction by human bronchial epithelial cells through TRPA1 and EGFR signaling. Toxicol Sci 174:278–290
Meng J, Li Y, Fischer MJM, Steinhoff M, Chen W, Wang J (2021) Th2 modulation of transient receptor potential channels: an unmet therapeutic intervention for atopic dermatitis. Front Immunol 12:696784
Mesquita G, Prevarskaya N, Schwab A, Lehen’kyi V (2021) Role of the TRP channels in pancreatic ductal adenocarcinoma development and progression. Cells 10:1021
Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB (2018) Regulation of pain and itch by TRP channels. Neurosci Bull 34:120–142
Moriya J (2019) Critical roles of inflammation in atherosclerosis. J Cardiol 73:22–27
Naert R, López-Requena A, Talavera K (2021) TRPA1 expression and pathophysiology in immune cells. Int J Mol Sci 22:11460
Nagatomo K, Kubo Y (2008) Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc Natl Acad Sci U S A 105:17373–17378
Nakagawa T, Kaneko S (2017) Roles of transient receptor potential ankyrin 1 in oxaliplatin-induced peripheral neuropathy. Biol Pharm Bull 40:947–953
Nassini R, Fusi C, Materazzi S, Coppi E, Tuccinardi T, Marone IM, De Logu F, Preti D, Tonello R, Chiarugi A et al (2015) The TRPA1 channel mediates the analgesic action of dipyrone and pyrazolone derivatives. Br J Pharmacol 172:3397–3411
Nilius B, Appendino G, Owsianik G (2012) The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch 464:425–458
Ohara K, Fukuda T, Ishida Y, Takahashi C, Ohya R, Katayama M, Uchida K, Tominaga M, Nagai K (2017) β-Eudesmol, an oxygenized sesquiterpene, stimulates appetite via TRPA1 and the autonomic nervous system. Sci Rep 7:15785
Özkal B, Övey İ S (2020) Correction to: selenium enhances TRPA1 channel-mediated activity of temozolomide in SH-SY5Y neuroblastoma cells. Child’s Nervous 36:1293
Panicker JN, Fowler CJ, Kessler TM (2015) Lower urinary tract dysfunction in the neurological patient: clinical assessment and management. Lancet Neurol 14:720–732
Paumier A, Boisseau S, Jacquier-Sarlin M, Pernet-Gallay K, Buisson A, Albrieux M (2022) Astrocyte-neuron interplay is critical for Alzheimer’s disease pathogenesis and is rescued by TRPA1 channel blockade. Brain 145:388–405
Philyppov IB, Paduraru ON, Gulak KL, Skryma R, Prevarskaya N, Shuba YM (2016) TRPA1-dependent regulation of bladder detrusor smooth muscle contractility in normal and type I diabetic rats. J Smooth Muscle Res 52:1–17
Rhyu MR, Kim Y, Lyall V (2021) Interactions between chemesthesis and taste: role of TRPA1 and TRPV1. Int J Mol Sci 22:3360
Russo AF (2015) Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol 55:533–552
Sairenji T, Collins KL, Evans DV (2017) An update on inflammatory bowel disease. Prim Care 44:673–692
Sanechika S, Shimobori C, Ohbuchi K (2021) Identification of herbal components as TRPA1 agonists and TRPM8 antagonists. J Nat Med 75:717–725
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM (2021) Alzheimer’s disease. Lancet (London) 397:1577–1590
Shang S, Zhu F, Liu B, Chai Z, Wu Q, Hu M, Wang Y, Huang R, Zhang X, Wu X et al (2016) Intracellular TRPA1 mediates Ca2+ release from lysosomes in dorsal root ganglion neurons. J Cell Biol 215:369–381
Shatillo A, Koroleva K, Giniatullina R, Naumenko N, Slastnikova AA, Aliev RR, Bart G, Atalay M, Gu C, Khazipov R et al (2013) Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience 253:341–349
Shi W, Li C, Wartmann T, Kahlert C, Du R, Perrakis A, Brunner T, Croner RS, Kahlert UD (2022) Sensory ion channel candidates inform on the clinical course of pancreatic cancer and present potential targets for repurposing of FDA-approved agents. J Personalized Med 12:478
Skerratt S (2017) Recent progress in the discovery and development of TRPA1 modulators. Prog Med Chem 56:81–115
Soria Lopez JA, González HM, Léger GC (2019) Alzheimer’s disease. Handb Clin Neurol 167:231–255
Soriano JB, Polverino F, Cosio BG (2018) What is early COPD and why is it important? Eur Respir J 52:1801448
Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, ME, L.L. (2013) Oxidative stress and cancer: an overview. Ageing Res Rev 12:376–390
Spix B, Jeridi A, Ansari M, Yildirim A, Schiller HB, Grimm C (2022) Endolysosomal cation channels and lung disease. Cells 11:304
Strassmaier T, Bakthavatchalam R (2011) Transient receptor potential A1 modulators. Curr Top Med Chem 11:2227–2236
Sura L, Zíma V, Marsakova L, Hynkova A, Barvík I, Vlachova V (2012) C-terminal acidic cluster is involved in Ca2+-induced regulation of human transient receptor potential ankyrin 1 channel. J Biol Chem 287:18067–18077
Takahashi N, Chen HY, Harris IS, Stover DG, Selfors LM, Bronson RT, Deraedt T, Cichowski K, Welm AL, Mori Y et al (2018) Cancer cells co-opt the neuronal redox-sensing channel TRPA1 to promote oxidative-stress tolerance. Cancer Cell 33:985-1003.e1007
Talavera K, Startek JB, Alvarez-Collazo J, Boonen B, Alpizar YA, Sanchez A, Naert R, Nilius B (2020) Mammalian transient receptor potential TRPA1 channels: from structure to disease. Physiol Rev 100:725–803
Üstünel L, Özgüler IM (2021) The effects of iloprost and beta3 receptor agonist on TRPA1 and TRPC1 immunreactivity in an experimental lower extremty ischemia-reperfusion injury model. Turk J Med Sci 51:2763–2770
Verkhratsky A, Nedergaard M (2018) Physiology of Astroglia. Physiol Rev 98:239–389
Von Molitor E, Riedel K, Hafner M, Rudolf R, Cesetti T (2020) Sensing senses: optical biosensors to study gustation. Sensors (Basel, Switzerland) 20:1811
Wan P, Su W, Zhang Y, Li Z, Deng C, Li J, Jiang N, Huang S, Long E, Zhuo Y (2020) LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury. Cell Death Differ 27:176–191
Wang S, Kobayashi K, Kogure Y, Yamanaka H, Yamamoto S, Yagi H, Noguchi K, Dai Y (2018) Negative regulation of TRPA1 by AMPK in primary sensory neurons as a potential mechanism of painful diabetic neuropathy. Diabetes 67:98–109
Wang M, Zhang Y, Xu M, Zhang H, Chen Y, Chung KF, Adcock IM, Li F (2019a) Roles of TRPA1 and TRPV1 in cigarette smoke -induced airway epithelial cell injury model. Free Radical Biol Med 134:229–238
Wang Z, Ye D, Ye J, Wang M, Liu J, Jiang H, Xu Y, Zhang J, Chen J, Wan J (2019b) The TRPA1 channel in the cardiovascular system: promising features and challenges. Front Pharmacol 10:1253
Wang Q, Chen K, Zhang F, Peng K, Wang Z, Yang D, Yang Y (2020) TRPA1 regulates macrophages phenotype plasticity and atherosclerosis progression. Atherosclerosis 301:44–53
Wang S, Wang B, Shang D, Zhang K, Yan X, Zhang X (2022) Ion channel dysfunction in astrocytes in neurodegenerative diseases. Front Physiol 13:814285
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, Li CY, Li CJ (2018) Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem 46:1650–1667
Wu CK, Lin JF, Lee TS, Kou YR, Tarng DC (2021a) Role of TRPA1 in tissue damage and kidney disease. Int J Mol Sci 22:3415
Wu CK, Wu CL, Lee TS, Kou YR, Tarng DC (2021b) Renal tubular epithelial TRPA1 acts as an oxidative stress sensor to mediate ischemia-reperfusion-induced kidney injury through MAPKs/NF-κB signaling. Int J Mol Sci 22:2309
Wu N, Chen D, Sun H, Tan J, Zhang Y, Zhang T, Han Y, Liu H, Ouyang X, Yang XD et al (2021c) MAP3K2 augments Th1 cell differentiation via IL-18 to promote T cell-mediated colitis. Sci China Life Sci 64:389–403
Yuan J, Liang X, Zhou W, Feng J, Wang Z, Shen S, Guan X, Zhao L, Deng F (2021) TRPA1 promotes cisplatin-induced nephrotoxicity through inflammation mediated by the MAPK/NF-κB signaling pathway. Ann Transl Med 9:1578
Zhang YZ, Li YY (2014) Inflammatory bowel disease: pathogenesis. World J Gastroenterol 20:91–99
Zhao JF, Shyue SK, Kou YR, Lu TM, Lee TS (2016) Transient receptor potential ankyrin 1 channel involved in atherosclerosis and macrophage-foam cell formation. Int J Biol Sci 12:812–823
Zhu R, Liu H, Liu C, Wang L, Ma R, Chen B, Li L, Niu J, Fu M, Zhang D et al (2017) Cinnamaldehyde in diabetes: a review of pharmacology, pharmacokinetics and safety. Pharmacol Res 122:78–89
Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA (2007) Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 10:277–279
Acknowledgements
We also thank Professor Biguang Tuo (Department of Gastroenterology, Affiliated Hospital of Zunyi Medical University) for highly professional services.
Funding
This study was supported by research grants the National Natural Science Foundation of China (No.81660099; No. 82170628; No.81970541; No.31960151; No.32160208; No.81770610), the Guizhou Science and Technology Department (Qiankehe platform talents (2021–5647)), the Guizhou Science and Technology Department (Qiankehe foundation-ZK (2021–major project 004)), the Zunyi Science and Technology Bureau (Outstanding Young Talents in Zunyi City (2018-9; 2020-1)), the Collaborative Innovation Center of Chinese Ministry of Education (2020-39), the Graduate Education and Teaching Innovation Program of Zunyi Medical University (ZYK55), and the Science and Technology Plan Project of Guizhou Province (QIAN KE HE JI CHU-ZK(2023)YI BAN556).
Author information
Authors and Affiliations
Contributions
JJL and HFZ wrote the manuscript. QD, JYG, JBW, and QL collect the literature. ZL revised the manuscript for clarity and style. TZ primarily revised and finalized manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical Approval
No ethics approval was required for this review that did not involve patients or patient data.
Consent for Publication
We have obtained consents to publish this paper from all the participants of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Zhang, H., Du, Q. et al. Research Progress on TRPA1 in Diseases. J Membrane Biol 256, 301–316 (2023). https://doi.org/10.1007/s00232-023-00277-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-023-00277-x