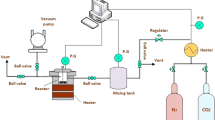
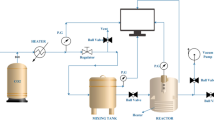
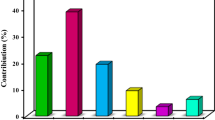
Abstract
In this work, the CO2 absorption by potassium hydroxide aqueous solution was studied. The response surface methodology (RSM) based on central composite design (CCD) was used to design experiments, make models, and find the optimum operating conditions for attaining desirable responses in the range of temperature, pressure and absorbent concentration of 25–65 °C, 2–10 bar and 0.01–1.21 mol/lit, respectively. The effects of process variables and their interactions on the responses were investigated with the numerical model, obtained from experimental data fitting to a second-order polynomial model, to achieve the optimal conditions. The experiments and numerical model indicated that the increase in temperature and absorbent concentration decrease CO2 loading, and an increase in pressure increase CO2 loading. Optimum conditions were found to be the temperature of 35 °C, pressures of 4 bar and, KOH concentration of 0.412 mol/lit. The CO2 loading of 0.745 and CO2 removal efficiency of 32.221% were achieved in the optimal conditions. The modified Pitzer’s GE model was used for CO2 + KOH + H2O system, in order to investigate the species concentration in the liquid phase. The average relative error between predicted CO2 loading and experimental CO2 loading was 7.4%.













Similar content being viewed by others
Notes
Monoethanolamine.
Piperazine.
Ethylenediamine.
Methyl diethanolamine.
Diethanolamine.
Electrolyte Non-Random Two-Liquid Model.
Abbreviations
- \({{R}}_{{{CO}}_{2}}\) :
-
CO2 removal efficiency (%)
- Pi :
-
Initial Pressure Pa
- Pf :
-
Final Pressure Pa
- Vg :
-
Volume of gas cm3
- T:
-
Temperature K
- C:
-
Absorbent concentration (mol)
- P:
-
Pressure (bar, Pa)
- R:
-
Universal gas constant (cm3Pa/K.mol)
- KR :
-
Equilibrium constant for the chemical reaction R, expressed on the molality scale (-)
- \({{H}}_{{{CO}}_{2},{{H}}_{2}{O}}\) :
-
Henry’s constant for the solubility of carbon dioxide in pure water (Pa.kg/mol)
- \({{V}}_{{{CO}}_{2},{{H}}_{2}{O}}^{\infty }\) :
-
Partial molar volumes of CO2 dissolved at infinite dilution in water (cm3/mol)
- \({{P}}_{i}^{{sat}}\) :
-
Saturated vapor pressure of component I (Pa)
- TC :
-
Critical temperature (K)
- PC :
-
Critical pressure (Pa)
- \({{B}}_{{i},{j}}\) :
-
Second virial coefficients between species i and j (cm3/mol)
- GE :
-
Excess Gibs free energy (J)
- \({{M}}_{{{H}}_{2}{O}}\) :
-
Molar mass of water (kg/mol0029
- I:
-
Ionic strength (kmol/m3)
- \({{A}}_{\varnothing}\) :
-
Debye-Hueckel parameter (-)
- Cϕ :
-
Third Virial coefficient in Pitzer’s model (Pa)
- C:
-
Absorbent concentration (mol/lit)
- \({V}_{{H}_{2}O}\) :
-
Partial molar volume (cm3/mol)
- Z:
-
Compressibility factor (-)
- ni :
-
Mole number of component I (mol)
- \({m}_{i}\) :
-
Molality of component i in the solution (mol/kg)
- \({m}_{i}^{0}\) :
-
Initial molality of component i in the solution (mol/kg)
- \({{y}}_{{i}}\) :
-
Mole fraction of component i in the gas phase (-)
- zi :
-
Charge of ion I (-)
- \({{\alpha }}_{{{CO}}_{2}}\) :
-
CO2 loading (-)
- \({\varphi }_{i}^{v}\) :
-
Fugacity coefficients of of component i in the gas phase (-)
- \({\alpha }_{{H}_{2}O}\) :
-
Activity of water (-)
- γi :
-
Activity coefficient of component i (-)
- \({\beta }_{i,j}^{\left(0\right)}, {\beta }_{i,j}^{\left(1\right)}\) :
-
Binary interaction parameters between species i and j in Pitzer's equation (-)
- \({\uptau }_{{ijk}}\) :
-
Ternary interaction parameter in Pitzer's equation (-)
- g:
-
Gas (-)
- C:
-
Critical (-)
- R:
-
Reaction R (-)
- ∞:
-
Infinite dilution in pure water (-)
- sat:
-
Saturation (-)
- \(v\) :
-
Gas phase (-)
References
Sreedhar I, Nahar T, Venugopal A, Srinivas B (2017) Carbon capture by absorption–path covered and ahead. Renew Sustain Energy Rev 76:1080–1107
Songolzadeh M, Soleimani M, Takht Ravanchi M, Songolzadeh R (2014) Carbon dioxide separation from flue gases: a technological review emphasizing reduction in greenhouse gas emissions. Sci World J 2014
Aaron D, Tsouris C (2005) Separation of CO2 from flue gas: a review. Sep Sci Technol 40(1–3):321–348
Bhown AS, Freeman BC (2011) Analysis and status of post-combustion carbon dioxide capture technologies. Environ Sci Technol 45(20):8624–8632
Koronaki I, Prentza L, Papaefthimiou V (2015) Modeling of CO2 capture via chemical absorption processes− An extensive literature review. Renew Sustain Energy Rev 50:547–566
Leung DY, Caramanna G, Maroto-Valer MM (2014) An overview of current status of carbon dioxide capture and storage technologies. Renew Sustain Energy Rev 39:426–443
Pashaei H, Ghaemi A, Nasiri M (2016) Modeling and experimental study on the solubility and mass transfer of CO 2 into aqueous DEA solution using a stirrer bubble column. RSC Adv 6(109):108075–108092
Pashaei H, Zarandi MN, Ghaemi A (2017) Experimental study and modeling of CO2 absorption into diethanolamine solutions using stirrer bubble column. Chem Eng Res Des 121:32–43
Svendsen HF, Hessen ET, Mejdell T (2011) Carbon dioxide capture by absorption, challenges and possibilities. Chem Eng J 171(3):718–724
Aschenbrenner O, Styring P (2010) Comparative study of solvent properties for carbon dioxide absorption. Energy Environ Sci 3(8):1106–1113
Gomes J, Santos S, Bordado J (2015) Choosing amine-based absorbents for CO2 capture. Environ Technol 36(1):19–25
Gouedard C, Picq D, Launay F, Carrette PL (2012) Amine degradation in CO2 capture. I. A review. International Journal of Greenhouse Gas Control 10: p. 244–270
Heydarifard M, Pashaei H, Ghaemi A, Nasiri M (2018) Reactive absorption of CO2 into Piperazine aqueous solution in a stirrer bubble column: Modeling and experimental. Int J Greenhouse Gas Control 79:91–116
Mirzaei F, Ghaemi A (2018) An experimental correlation for mass transfer flux of CO2 reactive absorption into aqueous MEA‐PZ blended solution. Asia Pac J Chem Eng 13(6):e2250
Masaki K (1931) on the CO2 absorption velocity of naoh-and koh-solutions. J Biochem 13(1):211–217
Pinsent B, Roughton F (1951) The kinetics of combination of carbon dioxide with water and hydroxide ions. Trans Faraday Soc 47:263–269
Pohorecki R, Moniuk W (1988) Kinetics of reaction between carbon dioxide and hydroxyl ions in aqueous electrolyte solutions. Chem Eng Sci 43(7):1677–1684
Rumpf B, Xia J, Maurer G (1998) Solubility of carbon dioxide in aqueous solutions containing acetic acid or sodium hydroxide in the temperature range from 313 to 433 K and at total pressures up to 10 MPa. Ind Eng Chem Res 37(5):2012–2019
Gondal S, Asif N, Svendsen HF, Knuutila H (2015) Kinetics of the absorption of carbon dioxide into aqueous hydroxides of lithium, sodium and potassium and blends of hydroxides and carbonates. Chem Eng Sci 123:487–499
Krauβ M, Rzehak R (2017) Reactive absorption of CO2 in NaOH: Detailed study of enhancement factor models. Chem Eng Sci 166:193–209
Lin C-C, Chu C-R (2015) Feasibility of carbon dioxide absorption by NaOH solution in a rotating packed bed with blade packings. Int J Greenhouse Gas Control 42:117–123
Yoo M, Han S-J, Wee J-H (2013) Carbon dioxide capture capacity of sodium hydroxide aqueous solution. J Environ Manage 114:512–519
Tirandazi B, Yahyaee A, Kianpour M, Shahhosseini S (2017) Experimental investigation and modeling of viscosity effect on carbon dioxide absorption using sodium hydroxide. J Environ Chem Eng 5(3):2597–2604
Gondal S (2014) Carbon dioxide absorption into hydroxide and carbonate systems
Azar C, Lindgren K, Obersteiner M, Riahi K, van Vuuren DP, den Elzen KMG, Möllersten K, Larson ED (2010) The feasibility of low CO2 concentration targets and the role of bio-energy with carbon capture and storage (BECCS). Clim Change 100(1):195–202
Yincheng G, Zhenqi N, Wenyi L (2011) Comparison of removal efficiencies of carbon dioxide between aqueous ammonia and NaOH solution in a fine spray column. Energy Procedia 4:512–518
Gondal S, Usman M, Monteiro JG, Svendsen HF, Knuutila H (2016) VLE Modeling of Aqueous Solutions of Unloaded and Loaded Hydroxides of Lithium. Sodium and Potassium Energy Procedia 86:282–293
Darmana D, Henket R, Deen N, Kuipers J (2007) Detailed modelling of hydrodynamics, mass transfer and chemical reactions in a bubble column using a discrete bubble model: Chemisorption of CO2 into NaOH solution, numerical and experimental study. Chem Eng Sci 62(9):2556–2575
Aroonwilas A, Tontiwachwuthikul P, Chakma A (2001) Effects of operating and design parameters on CO2 absorption in columns with structured packings. Sep Purif Technol 24(3):403–411
Javed K, Mahmud T, Purba E (2010) The CO2 capture performance of a high-intensity vortex spray scrubber. Chem Eng J 162(2):448–456
Peng Y, Zhao B, Li L (2012) Advance in post-combustion CO2 capture with alkaline solution: a brief review. Energy Procedia 14:1515–1522
Gondal S, Svendsen HF, Knuutila HK (2016) Activity based kinetics of CO2–OH− systems with Li+, Na+ and K+ counter ions. Chem Eng Sci 151:1–6
Haubrock J, Hogendoorn J, Versteeg GF (2005) The applicability of activities in kinetic expressions: a more fundamental approach to represent the kinetics of the system CO2-OH-in terms of activities. Int J Chem React Eng 3(1)
Kucka L, Richter J, Kenig E, Górak A (2003) Determination of gas–liquid reaction kinetics with a stirred cell reactor. Sep Purif Technol 31(2):163–175
Amiri M, Shahhosseini S, Ghaemi A (2017) Optimization of CO2 capture process from simulated flue gas by dry regenerable alkali metal carbonate based adsorbent using response surface methodology. Energy Fuels 31(5):5286–5296
Mohammad NK, Ghaemi A, Tahvildari K (2019) Hydroxide modified activated alumina as an adsorbent for CO2 adsorption: Experimental and modeling. Int J Greenhouse Gas Control 88:24–37
Saeidi M, Ghaemi A, Tahvildari K, Derakhshi P (2018) Exploiting response surface methodology (RSM) as a novel approach for the optimization of carbon dioxide adsorption by dry sodium hydroxide. J Chin Chem Soc 65(12):1465–1475
Kawazuishi K, Prausnitz JM (1987) Correlation of vapor-liquid equilibria for the system ammonia-carbon dioxide-water. Ind Eng Chem Res 26(7):1482–1485
Edwards T, Maurer G, Newman J, Prausnitz J (1978) Vapor-liquid equilibria in multicomponent aqueous solutions of volatile weak electrolytes. AIChE J 24(6):966–976
Dembo RS, Eisenstat SC, Steihaug T (1982) Inexact newton methods. SIAM J Numer Anal 19(2):400–408
Rumpf B, Maurer G (1993) An experimental and theoretical investigation on the solubility of carbon dioxide in aqueous solutions of strong electrolytes. Berichte der Bunsengesellschaft fuer physikalische chemie 97(1):85–97
Saul A, Wagner W (1987) International equations for the saturation properties of ordinary water substance. J Phys Chem Ref Data 16(4):893–901
Lewis G, Randall M (1961) Thermodynamics, ; Pitzer, KS; Brewer, L., Eds. McGraw-Hill: New York
He S, Morse JW (1993) The carbonic acid system and calcite solubility in aqueous Na-K-Ca-Mg-Cl-SO4 solutions from 0 to 90 C. Geochim Cosmochim Acta 57(15):3533–3554
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76(5):965–977
Mourabet M, El Rhilassi A, El Boujaady H, Bennani-Ziatni M, Taitai A (2017) Use of response surface methodology for optimization of fluoride adsorption in an aqueous solution by Brushite. Arab J Chem 10:S3292–S3302
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rastegar, Z., Ghaemi, A. CO2 absorption into potassium hydroxide aqueous solution: experimental and modeling. Heat Mass Transfer 58, 365–381 (2022). https://doi.org/10.1007/s00231-021-03115-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-021-03115-9