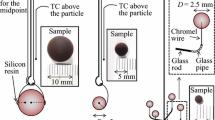
Abstract
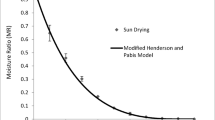
In this work, the model of particles for microwave drying by means of assisted force convection for blueberry leaves is described. A one-dimensional particle model is made in the direction of the thickness of the leaf. Only the phases of water during drying are considered. The mass and energy equation in the particle model develops. The effective diffusivity and the Arrhenius equation for the water phase (liquid and vapor) are considered in the mass equation. The energy equation considers the Lambert-Beer equation. The simulation is performed for different cases of microwave powers (100, 300, 400 W) and temperatures (50, 60 and 70 °C) The activation energy and the pre-exponential factor in the Arrhenius equation are taken from the kinetic analysis prior to this Work The temperature and mass profiles for some experimental and theoretical cases are compared, and it is observed that the model considered gives good results of adjustment between the experimental and the theoretical.








Similar content being viewed by others
References
Datta AK (2007) Porous media approaches to studying simultaneous heat and mass transfer in food processes. I: Problem formulations. J Food Eng 80:80–95. https://doi.org/10.1016/j.jfoodeng.2006.05.013
Warning AD, Arquiza JMR, Datta AK (2014) A multiphase porous medium transport model with distributed sublimation front to simulate vacuum freeze drying. Food Bioprod Process 94:637–648. https://doi.org/10.1016/j.fbp.2014.08.011
Ho QT, Carmeliet J, Datta AK, Defraeye T, Delele MA, Herremans E et al (2013) Multiscale modeling in food engineering. J Food Eng 114:279–291. https://doi.org/10.1016/j.jfoodeng.2012.08.019
Defraeye T (2014) Advanced computational modelling for drying processes - A review. Appl Energy 131:323–344. https://doi.org/10.1016/j.apenergy.2014.06.027
Kapellos GE, Alexiou TS, Payatakes AC (2012) A multiscale theoretical model for fluid flow in cellular biological media. Int J Eng Sci 51:241–271. https://doi.org/10.1016/j.ijengsci.2011.09.010
Fanta SW, Abera MK, Ho QT, Verboven P, Carmeliet J, Nicolai BM (2013) Microscale modeling of water transport in fruit tissue. J Food Eng 118:229–237. https://doi.org/10.1016/j.jfoodeng.2013.04.003
Karunasena HCP, Senadeera W, Brown RJ, Gu YT (2014) A particle based model to simulate microscale morphological changes of plant tissues during drying. Soft Matter 10:5249–5268. https://doi.org/10.1039/c4sm00526k
Karunasena HCP, Senadeera W, Brown RJ, Gu YT (2014) A meshfree model for plant tissue deformations during drying. ANZIAM J 55:110–113
Nam JH, Song CS (2007) Numerical simulation of conjugate heat and mass transfer during multi-dimensional freeze drying of slab-shaped food products. Int J Heat Mass Transf 50:4891–4900. https://doi.org/10.1016/j.ijheatmasstransfer.2007.08.004
Verboven P, Kerckhofs G, Mebatsion HK, Ho QT, Temst K, Wevers M et al (2008) Three-dimensional gas exchange pathways in pome fruit characterized by synchrotron x-ray computed tomography. Plant Physiol 147:518–527. https://doi.org/10.1104/pp.108.118935
Luikov AV (1968) Two-Dimensional temperature field: Particular problems. Anal Heat Diffus Theory 460:245–248. https://doi.org/10.1016/B978-0-12-459756-3.50016-X
Budd CJ (2011) Hill a. DC. A comparison of models and methods for simulating the microwave heating of moist foodstuffs. Int J Heat Mass Transf 54:807–817. https://doi.org/10.1016/j.ijheatmasstransfer.2010.10.022.
Zhao J, Zhang T, Corless RM (2006) Convergence of the compact finite difference method for second-order elliptic equations. Appl Math Comput 182:1454–1469. https://doi.org/10.1016/j.amc.2006.05.033
Leonard BP (1995) Order of accuracy of QUICK and related convection-diffusion schemes. Appl Math Model 19:640–653. https://doi.org/10.1016/0307-904X(95)00084-W
Pettermann HE, Huber CO, Luxner MH, Nogales S, Böhm HJ (2010) An incremental mori-tanaka homogenization scheme for finite: Strain thermoelastoplasticity of mmcs. Materials (Basel) 3:434–451. https://doi.org/10.3390/ma3010434
Karunasena HCP, Brown RJ, Gu YT, Senadeera W (2015) Application of meshfree methods to numerically simulate microscale deformations of different plant food materials during drying. J Food Eng 146:209–226. https://doi.org/10.1016/j.jfoodeng.2014.09.011
Yao Z, Le Maguer M (1997) Mathematical Modelling and Simulation of Mass lkansfer in Osmotic Dehydration Processes. Part III: Parametric Study. J Food Eng 8774:33–46. https://doi.org/10.1016/S0260-8774(97)00090-3
Chen D-SD, Singh RK, Haghighi K, Nelson PE (1993) Finite element analysis of temperature distribution in microwaved cylindrical potato tissue. J Food Eng 18:351–368. https://doi.org/10.1016/0260-8774(93)90052-L
Balaban M (1989) Effect of volume change in foods on the temperature and moisture content predictions of simultaneous heat and moisture transfer models. J Food Process Eng 12:67–88. https://doi.org/10.1111/j.1745-4530.1990.tb00041.x
Curcio S, Aversa M (2014) Influence of shrinkage on convective drying of fresh vegetables: A theoretical model. J Food Eng 123:36–49. https://doi.org/10.1016/j.jfoodeng.2013.09.014
Kowalski SJ (2002) Modelling of fracture phenomena in dried materials. Chem Eng J 86:145–151. https://doi.org/10.1016/S1385-8947(01)00282-0
Udell KS (1985) Heat transfer in porous media considering phase change and capillarity-the heat pipe effect. Int J Heat Mass Transf 28:485–495. https://doi.org/10.1016/0017-9310(85)90082-1
Campañone LA, Zaritzky NE (2005) Mathematical analysis of microwave heating process. J Food Eng 69:359–368. https://doi.org/10.1016/j.jfoodeng.2004.08.027
Doymaz I (2006) Thin-layer drying behaviour of mint leaves. J Food Eng 74:370–375. https://doi.org/10.1016/j.jfoodeng.2005.03.009
Doymaz I, Tugrul N, Pala M (2006) Drying characteristics of dill and parsley leaves. J Food Eng 77:559–565. https://doi.org/10.1016/j.jfoodeng.2005.06.070
Özbek B, Dadali G (2007) Thin-layer drying characteristics and modelling of mint leaves undergoing microwave treatment. J Food Eng 83:541–549. https://doi.org/10.1016/j.jfoodeng.2007.04.004
Onwude DI, Hashim N, Janius RB, Nawi NM, Abdan K (2016) Modeling the Thin-Layer Drying of Fruits and Vegetables: A Review. Compr Rev Food Sci Food Saf 15:599–618. https://doi.org/10.1111/1541-4337.12196
De VD (1958) Simultaneous transfer of heat and moisture in porous media. Trans Am Geophys Union 39:909–916
Datta AK (2007) Porous media approaches to studying simultaneous heat and mass transfer in food processes. II: Property data and representative results. J Food Eng 80:96–110. https://doi.org/10.1016/j.jfoodeng.2006.05.012
Batista LM, da Rosa CA, Pinto LAA (2007) Diffusive model with variable effective diffusivity considering shrinkage in thin layer drying of chitosan. J Food Eng 81:127–132. https://doi.org/10.1016/j.jfoodeng.2006.10.014
Chen XD, Sidhu H, Nelson M (2011) Theoretical probing of the phenomenon of the formation of the outermost surface layer of a multi-component particle, and the surface chemical composition after the rapid removal of water in spray drying. Chem Eng Sci 66:6375–6384. https://doi.org/10.1016/j.ces.2011.08.035
Aregawi W, Defraeye T, Saneinejad S, Vontobel P, Lehmann E, Carmeliet J et al (2013) Dehydration of apple tissue: Intercomparison of neutron tomography with numerical modelling. Int J Heat Mass Transf 67:173–182. https://doi.org/10.1016/j.ijheatmasstransfer.2013.08.017
Aregawi WA, Defraeye T, Verboven P, Herremans E, Roeck GD, Nicolai BM (2013) Modelling of coupled water transport and large deformation during dehydration of apple tissue. Food Bioprocess Technol 6:1963–1978. https://doi.org/10.1016/j.jtbi.2007.03.018
Erbay Z, Icier F (2010) A Review of Thin Layer Drying of Foods: Theory, Modeling, and Experimental Results. Crit Rev Food Sci Nutr 50:441–464. https://doi.org/10.1080/10408390802437063
Rayaguru K, Routray W (2011) Microwave drying kinetics and quality characteristics of aromatic Pandanus amaryllifolius leaves. Int Food Res J 18:1035–1042
Wang R, Zhou W, R-AH W (2006) Kinetic study of the thermal stability of tea catechins in aqueous systems using a microwave reactor. J Agric Food Chem 54:5924–5932. https://doi.org/10.1021/jf0611419
Dadalı G, Kılıç Apar D, Özbek B (2007) Microwave Drying Kinetics of Okra. Dry Technol 25:917–924. https://doi.org/10.1080/07373930701372254
Kasaoka S, Sakata Y, Shimada M, Matsutomi T (1985) New Kinetic Model for Temperature Programmed Thermogravimetry and Its Applications To the Gasification of Coal Chars With Steam and Carbon Dioxide. J Chem Eng Japan 18:426–432. https://doi.org/10.1252/jcej.18.426
Chizoba Ekezie FG, Sun DW, Han Z, Cheng JH (2017) Microwave-assisted food processing technologies for enhancing product quality and process efficiency: A review of recent developments. Trends Food Sci Technol 67:58–69. https://doi.org/10.1016/j.tifs.2017.05.014
Marra F, De Bonis MV, Ruocco G (2010) Combined microwaves and convection heating: A conjugate approach. J Food Eng 97:31–39. https://doi.org/10.1016/j.jfoodeng.2009.09.012
Talens C, Castro-Giraldez M, Fito PJ (2016) A thermodynamic model for hot air microwave drying of orange peel. J Food Eng 175:33–42. https://doi.org/10.1016/j.jfoodeng.2015.12.001
Salagnac P, Glouannec P, Lecharpentier D (2004) Numerical modeling of heat and mass transfer in porous medium during combined hot air, infrared and microwaves drying. Int J Heat Mass Transf 47:4479–4489. https://doi.org/10.1016/j.ijheatmasstransfer.2004.04.015
Venkatesh MS, Raghavan GSV (2004) An overview of microwave processing and dielectric properties of agri-food materials. Biosyst Eng 88:1–18. https://doi.org/10.1016/j.biosystemseng.2004.01.007
Whitaker S (1977) Simultaneous Heat, Mass, and Momentum Transfer in Porous Media: A Theory of Drying. Adv Heat Tran 13:119–203. https://doi.org/10.1016/S0065-2717(08)70223-5
Quintard M, Whitaker S (1993) Transport in ordered and disordered porous media: volume-averaged equations, closure problems, and comparison with experiment. Chem Eng Sci 48:2537–2564. https://doi.org/10.1016/0009-2509(93)80266-S
Li N, Taylor LS, Ferruzzi MG, Mauer LJ (2012) Kinetic Study of Catechin Stability: Effects of pH, Concentration, and Temperature. J Agric Food Chem 60:12531–12539. https://doi.org/10.1021/jf304116s
Joardder MUH, Karim A, Kumar C, Brown RJ (2015) Porosity: Establishing the Relationship between Drying Parameters and Dried Food Quality
Barbosa-Canovas GV (1996) Dehydration of foods. Chapman & Hall, New York
Liu JY, Shun C (1991) Solutions of Luikov equations of heat and mass transfer in capillary-porous bodies. Int J Heat Mass Transf 34:1747–1754. https://doi.org/10.1016/0017-9310(91)90150-D
Halder A, Datta AK (2012) Surface heat and mass transfer coefficients for multiphase porous media transport models with rapid evaporation. Food Bioprod Process 90:475–490. https://doi.org/10.1016/j.fbp.2011.10.005
Puangsuwan K, Chongcheawchamnan M, Tongurai C (2015) Effective Moisture Diffusivity, Activation Energy and Dielectric Model for Palm Fruit Using a Microwave Heating. J Microw Power Electromagn Energy 49:100–111. https://doi.org/10.1080/08327823.2015.11689900
Nield DA, Bejan A (2017) Convection in Porous Media. 5 ed. Switzerland. Springer-Verlag, Berlin Heidelberg. https://doi.org/10.1007/978-3-319-49562-0.
Khawam A, Flanagan DR (2006) Solid-State Kinetic Models: Basics and Mathematical Fundamentals. J Phys Chem 110:17315–17328. https://doi.org/10.1021/jp062746a
Chandrasekaran S, Ramanathan S, Basak T (2013) Microwave food processing-A review. Food Res Int 52:243–261. https://doi.org/10.1016/j.foodres.2013.02.033
Yang J, Di Q, Zhao J, Wang L (2009) Mechanism on mass transfer in micro-scale during the microwave drying of plant porous materials. Proc ASME Summer Heat Transf Conf, HT2009 3:8–10. https://doi.org/10.1115/HT2009-88389
Yang J, Chen JF, Zhao YY, Mao LC (2010) Effects of drying processes on the antioxidant properties in sweet potatoes. Agric Sci China 9:1522–1529. https://doi.org/10.1016/S1671-2927(09)60246-7
Balaban M, Piggot GM (1988) Mathematical model of simultaneous heat and mass transfer in food with dimensional changes and variable transport parameters. J Food Sci 53:935–939. https://doi.org/10.1111/j.1365-2621.1988.tb08990.x
Hasatani M, Itaya Y, Miura K (1988) Hybrid Drying of Granular Materials By Combined Radiative and Convective Heating. Dry Technol 6:43–68. https://doi.org/10.1080/07373938808916360
Dhib R (2007) Infrared Drying: From Process Modeling to Advanced Process Control. Dry Technol 25:97–105. https://doi.org/10.1080/07373930601160908
Shafiur Rahman M (2009) Food propertries handbook. Second edi. Boca Raton
Esveld DC, Van Der Sman RGM, Van Dalen G, Van Duynhoven JPM, Meinders MBJ (2012) Effect of morphology on water sorption in cellular solid foods. Part I: Pore scale network model. J Food Eng 109:301–310. https://doi.org/10.1016/j.jfoodeng.2011.08.016
Esveld DC, Van Der Sman RGM, Witek MM, Windt CW, Van AH, Van Duynhoven JPM et al (2012) Effect of morphology on water sorption in cellular solid foods. Part II: Sorption in cereal crackers. J Food Eng 109:311–320. https://doi.org/10.1016/j.jfoodeng.2011.08.023
Nguyen TA, Verboven P, Scheerlinck N, Veraverbeke E, Nicolai BM (2003) An estimation procedure of effective diffusivity in pear tissue by means of a numerical water diffusion model. Acta Hortic 599:541–548. https://doi.org/10.1016/j.jfoodeng.2004.11.019
Feng H, Yin Y, Tang J (2012) Microwave Drying of Food and Agricultural Materials: Basics and Heat and Mass Transfer Modeling. Food Eng Rev 4:89–106. https://doi.org/10.1007/s12393-012-9048-x
Syamaladevi RM, Sablani SS, Tang J, Powers J, Swanson BG (2009) State diagram and water adsorption isotherm of raspberry (Rubus idaeus). J Food Eng 91:460–467. https://doi.org/10.1016/j.jfoodeng.2008.09.025
Botheju WS, Amarathunge KSP, Mohamed MTZ (2008) Modeling moisture desorption isotherms and thermodynamic properties of fermented tea Dhool (Camellia sinensis var. assamica). Dry Technol 26:1294–1299. https://doi.org/10.1080/07373930802307324
Knani S, Aouaini F, Bahloul N, Khalfaoui M, Hachicha MA, Ben Lamine A et al (2014) Modeling of adsorption isotherms of water vapor on Tunisian olive leaves using statistical mechanical formulation. Phys A Stat Mech Its Appl 400:57–70. https://doi.org/10.1016/j.physa.2014.01.006
Santos MV, Vampa V, Califano A, Zaritzky N (2010) Numerical simulations of chilling and freezing processes applied to bakery products in irregularly 3D geometries. J Food Eng 100:32–42. https://doi.org/10.1016/j.jfoodeng.2010.03.024
Radhika GB, Shyma SM (2014) Estimation of Mass Transfer Parameters and Drying Characteristics of Black Pepper using Microwave Energy. Int J Innov Res Adv Eng 1:228–234
Zanoelo EF (2007) A theoretical and experimental study of simultaneous heat and mass transport resistances in a shallow fluidized bed dryer of mate leaves. Chem Eng Process Process Intensif 46:1365–1375. https://doi.org/10.1016/j.cep.2006.10.016
Bergman TL, Lavine A, Incropera FP (2017) Fundamentals of heat and mass transfer. John Wiley & Sons, Inc, Hoboken
Krokida MK, Maroulis ZB (1997) Effect of drying method on shrinkage and porosity. Dry Technol 15:2441–2458. https://doi.org/10.1080/07373939808917488
Rahman MS (2001) Toward Prediction of Porosity in Foods During Drying: a Brief Review. Dry Technol 19:1–13. https://doi.org/10.1081/DRT-100001349
Kajishima T, Taira K (2017) Computational Fluid Dynamics. Cambridge. Cambridge University Press, New York. https://doi.org/10.1007/978-3-319-45304-0.
Patankar SV (1980) Numerical heat transfer and fluid flow. Hemisphere Pub. Corp.; McGraw-Hill, Washington; New York
Allanic N, Salagnac P, Glouannec P (2007) Convective and radiant drying of a polymer aqueous solution. Heat Mass Transf Und Stoffuebertragung 43:1087–1095. https://doi.org/10.1007/s00231-006-0196-5
Rahman MS (2014) Mass–Volume–Area-Related Properties of Foods. In: Group T& F, editor. Eng. Prop. Foods. Fourth Edi, CRC Press, p. 1–36.
Acknowledgements
V.H. Borda-Yepes wish to thank the Colombian Administrative Department of Science, Technology and Innovation (COLCIENCIAS, #617) (Departamento Administrativo de Ciencia, Tecnología e Innovacion) for financial support awarded to the program Doctoral in Engineering - Energy System of the National University of Colombia, Sede Medellin, and to the stay of doctoral training at McGill University. F. Chejne wish to thank to the project “Strategy of transformation of the Colombian energy sector in the horizon 2030” funded by the call 788 of Colciencias: Scientific Ecosystem. Contract number FP44842-210-2018.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Annex 1: Analysis mathematical particle model
The balance of the mass equation in a biomass can be represented by the substantial derivative (20):
The mass m of Equation (21) can be expressed in terms of its density ρ and volume V and write the equation (22)
If we assume that the biomass does not move, that its velocity v = 0 its derivate \( \frac{\partial v}{\partial x}=0 \) of the equation (23) our that expression as:
the equation V and by including the term as the volumetric reaction By dividing by volume V the equation (24) and by including the therm as the volumentric reaction \( {r_{\varphi}}^{\hbox{'}\hbox{'}\hbox{'}}=\frac{r_{\varphi }}{V} \) it express:
The density ρ may be represent in the solid, liquid and gas phases terms with its respectivity porosity ε and volumetric fraction γ as the equation description (5)
The equation (26) represent the general balance of the mass in the terms of the desnsity ρ, the porosity γ, the volumetric fraction γ and chemical reactions rφ' ' '.
Annex 2: Thermal properties analysis on the leaf
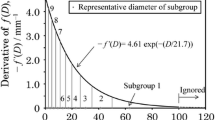
In the thermal properties were measured with KD2Pro device [62]. The conductivity thermal, diffusivity thermal, volume-specify heat capacity, and volumetric heat.

Time [min] | Power [W] | Temperature [C] | v [m/s] | Area [cm2] | Thin [mm] | L0 [mm] | Deff [m2/s] | kt [W/m K] | Pr | Re | Sc | Nu | Sh | ht W/m^2 K | hm m/s | Bih | Bim |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
0 | 100 | 50 | 0,5 | 7,678 | 0,50 | 30 | 2,00E-10 | 0,445 | 0,703 | 812,1 | 80870 | 16,8 | 818,3 | 249,2 | 5,46E-06 | 16,8 | 818,3 |
30 | 100 | 50 | 0,5 | 7,355 | 0,40 | 30 | 2,00E-10 | 0,445 | 0,703 | 812,1 | 80870 | 16,8 | 818,3 | 249,2 | 5,46E-06 | 16,8 | 818,3 |
60 | 100 | 50 | 0,5 | 7,099 | 0,25 | 30 | 2,00E-10 | 0,445 | 0,703 | 812,1 | 80870 | 16,8 | 818,3 | 249,2 | 5,46E-06 | 16,8 | 818,3 |
90 | 100 | 50 | 0,5 | 6,829 | 0,20 | 30 | 2,00E-10 | 0,445 | 0,703 | 812,1 | 80870 | 16,8 | 818,3 | 249,2 | 5,46E-06 | 16,8 | 818,3 |
0 | 300 | 60 | 0,5 | 8,398 | 0,50 | 30 | 2,00E-10 | 0,670 | 0,701 | 770,4 | 9,24E+04 | 16,4 | 833,1 | 365,6 | 5,55E-06 | 16,4 | 833,1 |
15 | 300 | 60 | 0,5 | 8,091 | 0,35 | 30 | 2,00E-10 | 0,670 | 0,701 | 770,4 | 9,24E+04 | 16,4 | 833,1 | 365,6 | 5,55E-06 | 16,4 | 833,1 |
30 | 300 | 60 | 0,5 | 7,680 | 0,25 | 30 | 2,00E-10 | 0,670 | 0,701 | 770,4 | 9,24E+04 | 16,4 | 833,1 | 365,6 | 5,55E-06 | 16,4 | 833,1 |
45 | 300 | 60 | 0,5 | 7,400 | 0,20 | 30 | 2,00E-10 | 0,670 | 0,701 | 770,4 | 9,24E+04 | 16,4 | 833,1 | 365,6 | 5,55E-06 | 16,4 | 833,1 |
0 | 400 | 70 | 0,5 | 8,026 | 0,50 | 30 | 2,00E-10 | 1,064 | 0,699 | 732,8 | 1,02E+05 | 15,9 | 840,8 | 565,7 | 5,61E-06 | 15,9 | 840,8 |
5 | 400 | 70 | 0,5 | 7,890 | 0,35 | 30 | 2,00E-10 | 1,064 | 0,699 | 732,8 | 9,24E+04 | 15,9 | 812,5 | 565,7 | 5,42E-06 | 15,9 | 812,5 |
10 | 400 | 70 | 0,5 | 7,601 | 0,25 | 30 | 2,00E-10 | 1,064 | 0,699 | 732,8 | 9,24E+04 | 15,9 | 812,5 | 565,7 | 5,42E-06 | 15,9 | 812,5 |
15 | 400 | 70 | 0,5 | 7,535 | 0,20 | 30 | 2,00E-10 | 1,064 | 0,699 | 732,8 | 9,24E+04 | 15,9 | 812,5 | 565,7 | 5,42E-06 | 15,9 | 812,5 |
Annex 3: Dielectric properties for differents temperatures and 100W powers

Rights and permissions
About this article
Cite this article
Borda-Yepes, V.H., Chejne, F., Granados, D.A. et al. Mathematical particle model for microwave drying of leaves. Heat Mass Transfer 55, 2959–2974 (2019). https://doi.org/10.1007/s00231-019-02626-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-019-02626-w