Abstract
Purpose
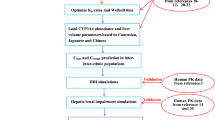
To develop a mathematical model combined between physiologically based pharmacokinetic and BTK occupancy (PBPK-BO) to simultaneously predict pharmacokinetic (PK) and pharmacodynamic (PD) changes of acalabrutinib (ACA) and active metabolite ACP-5862 in healthy humans as well as PD in patients. Next, to use the PBPK-BO to determine the optimal dosing regimens in patients alone, with different CYP3A4 variants, when co-administration with four CYP3A4 modulators and in patients with hepatic impairment, respectively.
Methods
The PBPK-BO model was built using physicochemical and biochemical properties of ACA and ACP-5862 and then verified by observed PK and PD data from healthy humans and patients. Finally, the model was applied to determine optimal dosing regimens in various clinical situations.
Results
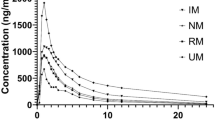
The simulations demonstrated that 100 mg ACA twice daily (BID) was the optimal dosing regimen in patients alone. Additionally, dosage regimens might be reduced to 50 mg BID in patients with five CYP3A4 variants. Moreover, the dosing regimen should be modified to 100 mg (even to 50 mg) once daily (QD) when co-administration with erythromycin or clarithromycin, and be increased to 200 mg BID with rifampicin, and but be avoided co-administration with itraconazole. Furthermore, dosage regimen simulations showed that optimal dosing might be decreased to 50 mg BID in patients with mild and moderate hepatic impairment, and be avoided taking ACA in severely hepatically impaired patients.
Conclusion
This PBPK-BO model can predict PK and PD in healthy humans and patients and also predict the optimal dosing regimens in various clinical situations.






Similar content being viewed by others
References
Markham A Dhillon S (2018) Acalabrutinib: first global approval. Drugs 78(1):139–145. https://doi.org/10.1007/s40265-017-0852-8
Khan Y, O’Brien S (2018) Acalabrutinib and its use in treatment of chronic lymphocytic leukemia. Future Oncol 15(6):579–589. https://doi.org/10.2217/fon-2018-0637
Podoll T et al (2019) Bioavailability, biotransformation, and excretion of the covalent Bruton tyrosine kinase inhibitor acalabrutinib in rats, dogs, and humans. Drug Metab Dispos 47(2):145–154. https://doi.org/10.1124/dmd.118.084459
US Food & Drug Administration. FDA approves Calquence (acalabrutinib). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/210259Orig1s000MultidisciplineR.pdf. Accessed 5 Jun 2022
Kaptein A et al (2019) AM2019-2194:Preclinical pharmacological profiling of ACP-5862, the major metabolite of the covalent BTK inhibitor acalabrutinib, displays intrinsic BTK inhibitory activity. Cancer Res 79(13):2194. https://doi.org/10.1158/1538-7445.AM2019-2194
Pepin XJH et al (2019) Bridging in vitro dissolution and in vivo exposure for acalabrutinib. Part II. A mechanistic PBPK model for IR formulation comparison, proton pump inhibitor drug interactions, and administration with acidic juices. Eur J Pharm Biopharm 142:435–448. https://doi.org/10.1016/j.ejpb.2019.07.011
Edlund H et al (2019) Population pharmacokinetics of the BTK inhibitor acalabrutinib and its active metabolite in healthy volunteers and patients with B-cell malignancies. Clin Pharmacokinet 58(5):659–672. https://doi.org/10.1007/s40262-018-0725-7
Fohner AE et al (2021) Characterization of CYP3A pharmacogenetic variation in American Indian and Alaska Native communities, targeting CYP3A4*1G allele function. Clin Transl Sci 14(4):1292–1302. https://doi.org/10.1111/cts.12970
Han M et al (2021) Functional assessment of the effects of CYP3A4 variants on acalabrutinib metabolism in vitro. Chem Biol Interact 345:109559. https://doi.org/10.1016/j.cbi.2021.109559
Alsadhan AA et al (2018) Variable Bruton tyrosine kinase (BTK) resynthesis across patients with chronic lymphocytic leukemia (CLL) on acalabrutinib therapy affect target occupancy and reactivation of B-cell receptor (BCR) signaling. Blood 132(Supplement 1):4401–4401. https://doi.org/10.1182/blood-2018-99-110391
Sun C et al (2020) Clinical and biological implications of target occupancy in CLL treated with the BTK inhibitor acalabrutinib. Blood 136(1):93–105. https://doi.org/10.1182/blood.2019003715
Zhou D et al (2019) Evaluation of the drug-drug interaction potential of acalabrutinib and its active metabolite, ACP-5862, using a physiologically-based pharmacokinetic modeling approach. CPT Pharmacometrics Syst Pharmacol 8(7):489–499. https://doi.org/10.1002/psp4.12408
Pepin XJH et al (2019) Bridging in vitro dissolution and in vivo exposure for acalabrutinib. Part I. Mechanistic modelling of drug product dissolution to derive a P-PSD for PBPK model input. Eur J Pharm Biopharm 142:421–434. https://doi.org/10.1016/j.ejpb.2019.07.014
Drozdzik M et al (2019) Protein abundance of clinically relevant drug transporters in the human liver and intestine: a comparative analysis in paired tissue specimens. Clin Pharmacol Ther 105(5):1204–1212. https://doi.org/10.1002/cpt.1301
Inoue T et al (2019) A new method to determine drug-target residence time of kinase inhibitors in living cells. Mole Cancer Ther 18(12). https://doi.org/10.1158/1535-7163.TARG-19-C085
Mathieson T et al (2018) Systematic analysis of protein turnover in primary cells. Nat Commun 9(1):689. https://doi.org/10.1038/s41467-018-03106-1
Heimbach T et al (2021) Physiologically-based pharmacokinetic modeling in renal and hepatic impairment populations: a pharmaceutical industry perspective. Clin Pharmacol Ther 110(2):297–310. https://doi.org/10.1002/cpt.2125
Willmann S et al (2021) Applications of physiologically based pharmacokinetic modeling of rivaroxaban-renal and hepatic impairment and drug-drug interaction potential. J Clin Pharmacol 61(5):656–665. https://doi.org/10.1002/jcph.1784
Simulations-Plus (2019) GastroPlus Manual 9.7
Qian CQ et al (2019) Simultaneously predict pharmacokinetic interaction of rifampicin with oral versus intravenous substrates of cytochrome P450 3A/Pglycoprotein to healthy human using a semi-physiologically based pharmacokinetic model involving both enzyme and transporter turnover. Eur J Pharm Sci 134:194–204. https://doi.org/10.1016/j.ejps.2019.04.026
Brown RP et al (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13(4):407–484. https://doi.org/10.1177/074823379701300401
Abdeldayem AR, Yasir S, Constantinescu SN, Moriggl R, Gunning PT (2020) Advances in covalent kinase inhibitors. Chem Soc Rev 49(9):2617–2687. https://doi.org/10.1039/c9cs00720b
Berry L, Zhao Z (2008) An examination of IC50 and IC50-shift experiments in assessing time-dependent inhibition of CYP3A4, CYP2D6 and CYP2C9 in human liver microsomes. Drug Metab Lett 2(1):51–59. https://doi.org/10.2174/187231208783478407
Bull HG et al (1996) Mechanism-based inhibition of human steroid 5α-reductase by finasteride: enzyme-catalyzed formation of NADP−dihydrofinasteride, a potent bisubstrate analog inhibitor. J Am Chem Soc 118(10 ):2359–2365. https://doi.org/10.1021/ja953069t
Li G et al (2021) Effect of CYP3A4 inhibitors and inducers on [harmacokinetics and pharmacodynamics of Saxagliptin and active metabolite M2 in humans using physiological-based pharmacokinetic combined DPP-4 occupancy. Front Pharmacol 12:746594. https://doi.org/10.3389/fphar.2021.746594
Gerner B, Scherf-Clavel O (2021) Physiologically based pharmacokinetic modelling of cabozantinib to simulate enterohepatic recirculation, drug-drug interaction with rifampin and liver impairment. Pharmaceutics 13(6). https://doi.org/10.3390/pharmaceutics13060778
Johnson DTN et al (2010) A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet 49(3):189–206. https://doi.org/10.2165/11318160-000000000-00000
Daryaee F et al (2017) A quantitative mechanistic PK/PD model directly connects Btk target engagement and in vivo efficacy. Chem Sci 8(5):3434–3443. https://doi.org/10.1039/c6sc03306g
Li X et al (2020) A physiologically based pharmacokinetic model of voriconazole integrating time-dependent inhibition of CYP3A4, genetic polymorphisms of CYP2C19 and predictions of drug-drug interactions. Clin Pharmacokinet 59(6):781–808. https://doi.org/10.1007/s40262-019-00856-z
AstraZeneca Pharmaceuticals. CALQUENCE (acalabrutinib capsule,gelatin coated): prescribing information. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dd4835ef-e1bc-4997-a399-1ffa2556fbfe. Accessed 5 Jun 2022
Barf T, Kaptein A (2012) Irreversible protein kinase inhibitors: balancing the benefits and risks. J Med Chem 55(14):6243–6262. https://doi.org/10.1021/jm3003203
Daryaee F, Tonge PJ (2019) Pharmacokinetic-pharmacodynamic models that incorporate drug-target binding kinetics. Curr Opin Chem Biol 50:120–127. https://doi.org/10.1016/j.cbpa.2019.03.008
Edlund H et al (2021) Improved characterization of the pharmacokinetics of acalabrutinib and its pharmacologically active metabolite, ACP-5862, in patients with B-cell malignancies and in healthy subjects using a population pharmacokinetic approach. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.14988
Edlund H et al (2021) Exposure-response analysis of acalabrutinib and its active metabolite ACP-5862 in patients with B-cell malignancies. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.15087
Saeheng T et al (2020) Physiologically-based pharmacokinetic modeling for optimal dosage prediction of quinine coadministered with ritonavir-boosted lopinavir. Clin Pharmacol Ther 107(5):1209–1220. https://doi.org/10.1002/cpt.1721
Zhang M et al (2019) Prediction of ticagrelor and its active metabolite in liver cirrhosis populations using a physiologically based pharmacokinetic model involving pharmacodynamics. J Pharm Sci 108(8):2781–2790. https://doi.org/10.1016/j.xphs.2019.03.028
Author information
Authors and Affiliations
Contributions
Guopeng Wang, Yang Liu, and Bowen Yi: conceptualization, formal analysis, supervision; Lifang Xu, Shuang Yu, and Huining Liu: investigation, resources, software, data curation, methodology, validation, visualization; Guopeng Wang, Lifang Xu, Shuang Yu, and Huining Liu: writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, L., Yu, S., Liu, H. et al. Physiologically based pharmacokinetic combined BTK occupancy modeling for optimal dosing regimen prediction of acalabrutinib in patients alone, with different CYP3A4 variants, co-administered with CYP3A4 modulators and with hepatic impairment. Eur J Clin Pharmacol 78, 1435–1446 (2022). https://doi.org/10.1007/s00228-022-03338-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03338-7