Abstract
Purpose
Ginkgo terpene lactones meglumine injection (GMI) is a novel preparation of traditional Chinese medicine that contains ginkgolides A, B and K (GA, GB, GK, respectively) as its primary components. In this study we evaluated the safety, tolerability and pharmacokinetics of these three ginkgolides after single and multiple intravenous infusions of GMI. We also investigated the effect of GMI on cytochrome P450 3A4 (CYP3A4) in healthy Chinese volunteers.
Methods
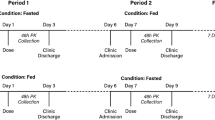
In this open-label, placebo-controlled study 15 subjects were randomly assigned to receive GMI or matched placebo (4:1 ratio). All subjects first received midazolam (MDZ) on day 1, followed by a 6-day washout. On Day 8, the subjects were started on once-daily dosing of either GMI or placebo for 14 days. Lastly, on Day 22 the subjects were given second dose of MDZ + GMI or MDZ + placebo. Plasma concentrations of ginkgolides, MDZ and its metabolite 1-hydroxy midazolam were quantified.
Results
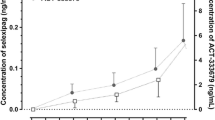
The steady-state conditions of GA, GB and GK were achieved after 6 days of daily dosing. Following a single dose of GMI (Day 8) the area under the concentration–timecurve from zero to 24 h after administration (AUC0-24h) of GA, GB and GK (arithmetic ± standard deviation) was 4.10 ± 1.06, 4.61 ± 1.31 and 0.127 ± 0.102 h μg/mL, respectively; the corresponding values following multiple doses of GMI (Day 19) were 3.94 ± 1.16, 5.00 ± 1.55 and 0.118 ± 0.096 h μg/mL, respectively. The mean accumulation ratios were 0.95, 1.08 and 0.89 for GA, GB and GK, respectively. Additionally, the geometric mean [peak concentration (Cmax) and AUC0-24h] ratios of MDZ and 1-hydroxy midazolam were all within the specified acceptance ranges in the MDZ + placebo treatment and MDZ + GMI treatment.
Conclusions
Our results show that GMI was well tolerated during the entire study. There was no systemic accumulation and no significant effects on the pharmacokinetics of MDZ in healthy Chinese male subjects after repeated dosing of GMI.




Similar content being viewed by others
References
Zhao B, Wang Z, Ling Y, Zhou S, Wu S, Dong S, Xie Y, Li L, Sun Y, Wang J, Ji H, Yan Y, Li Y, Guo J, Tian T, Xiao W (2013) Phase III clinical trial of Diterpene Ginkgolides Meglumine Injection for syndrome of stagnant phlegm blocking collaterals in convalescence of atherosclerotic thrombotic cerebral infarction. Chin Tradit Herbal Drugs 44(24):3525–30
Zhao B, Wang Z, Ling Y, Zhou S, Wu S, Dong S, Xie Y, Li L, Sun Y, Wang J, Ji H, Yan Y, Li Y, Guo J, Tian T, Xiao W (2013) Phase III clinical trial of Diterpene Ginkgolides Meglumine Injection for syndrome of stagnant phlegm blocking collaterals in convalescence of atherosclerotic thrombotic cerebral infarction. Chin Tradit Herbal Drugs 44(24):3525–30
Chen C, Zhou J, Chen J, Chang X, Zhang L, Wang Z (2014) Protective effects of ginkgo terpene lactones meglumine injection on focal cerebral ischemia reperfusion injury in rats. Chin J Exp Tradit Med Form 20(17):133–6
Mahadevan S, Park Y (2008) Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci 73(1):R14–19
Chan PC, Xia Q, Fu PP (2007) Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 25(3):211–44
Ma S, Liu X, Xun Q, Zhang X (2014) Neuroprotective effect of ginkgolide K against H2O2-induced PC12 cell cytotoxicity by ameliorating mitochondrial dysfunction and oxidative stress introduction. Biol Pharm Bull 37(2):217–25
Zheng B, Xing G, Bi Y, Yan G, Wang J, Cheng Y, Liu Y, Ashraf MA, Xie J (2016) Comparative pharmacokinetics of a proliposome formulation of Ginkgo biloba extract and Ginaton in rats by a sensitive ultra performance liquid chromatography-tandem mass spectrometry method. Saudi J Biol Sci 23(1):54–65
Li J, Li D, Hu J, Bi Y, Xiao W, Wang Z (2015) Simultaneous determination of ginkgolides A, B, C and bilobalide by LC-MS/MS and its application to a pharmacokinetic study in rats. Biomed Chromatogr 29(12):1907–12
Mooiman KD, Maas-Bakker RF, Hendrikx JJ, Bank PC, Rosing H, Beijnen JH, Schellens JH, Meijerman I (2014) The effect of complementary and alternative medicines on CYP3A4-mediated metabolism of three different substrates: 7-benzyloxy-4-trifluoromethyl-coumarin, midazolamand docetaxel. J Pharm Pharmacol l66(6):865–74
Zhou W, Ruigrok TJ (1990) Protective effect of Danshen during myocardial ischemia and reperfusion: an isolated rat heart study. Am J Chin Med 18(1–2):19–24
Zou L, Harkey MR, Henderson GL (2002) Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci 71(13):1579–89
Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, Ang CY (2002) Cytochrome P450 phenotypic ratios for predicting herb-drug interactions inhumans. Clin Pharmacol Ther 72(3):276–87
Smith M, Lin KM, Zheng YP (2001) An open trial of nifedipine-herb interactions with St. John’s wort, ginseng, and ginkgo biloba. Clin Pharmacol Ther 69:86 (abstract)
Garrett M, Smeraglia J, Lin X, Tan L, Tran J (2005) A pilot study to assess simultaneous administration of oral midazolam (MDZ) and fexofenadine (FEX) for the evaluation of cytochrome (CYP) 3A4 and P-glycoprotein (P-gp) activities. Clin Pharmacol Ther 77:45
Seo KA, Bae SK, Choi YK, Choi CS, Liu KH, Shin JG (2010) Metabolism of 1′- and 4-hydroxymidazolam by glucuronide conjugation is largely mediated by UDP-glucuronosyl transferases 1A4, 2B4, and 2B7. Drug Metab Dispos 38(11):2007–13
Lee JI, Chaves-Cnecco D, Amico JA, Kroboth PD, Wilson JW, Frye RF (2002) Application of semisimultaneous midazolam administration for hepatic and intestinal cytochrome P450 3A phenotyping. Clin Pharmacol Ther 72(6):718–28
Zadoyan G, Rokitta D, Klement S, Dienel A, Hoerr R, Gramatté T, Fuhr U (2012) Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol 68(5):553–60
Gaudineau C, Beckerman R, Welbourn S, Auclair K (2004) Inhibition of human P450 enzymes by multiple constituents of the Ginkgo biloba extract. Biochem Biophys Res Commun 318(4):1072–8
Wang S, Ouyang B, Aa J, Geng J, Fei F, Wang P, Wang J, Peng Y, Geng T, Li Y, Huang W, Wang Z, Xiao W, Wang G (2016) Pharmacokinetics and tissue distribution of ginkgolide A, ginkgolide B, and ginkgolide K after intravenous infusion of ginkgo diterpene lactones in a rat model. J Pharm Biomed Anal 126:109–16
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Shao, F., Zhang, H., Xie, L. et al. Pharmacokinetics of ginkgolides A, B and K after single and multiple intravenous infusions and their interactions with midazolam in healthy Chinese male subjects. Eur J Clin Pharmacol 73, 537–546 (2017). https://doi.org/10.1007/s00228-017-2197-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2197-3