Abstract
For anadromous fish entering the marine environment, we expect the probability of avoiding predation and starvation to increase with the quality and/or quantity of dietary resources consumed during the period immediately prior to, and following, ocean entry. Here, we report the results of research examining trophic history in relation to fork length, mass, and body condition in juvenile Chinook salmon captured in the southern Bering Sea using δ13C and δ15N analysis of skeletal muscle and liver samples. Our results show little inter-individual variability in δ15N, but variability in δ13C among tissues and within and among years was observed. Further, we found few relationships between δ15N and morphological or condition metrics, but strong relationships between δ13C and fork length, body mass, and Fulton’s K. We attribute the similarity in δ15N among individuals to high trophic level feeding (i.e., piscivory) associated with the prolonged duration of freshwater residency observed for juvenile Chinook salmon in our study area. Variation in δ13C, as well as relationships between δ13C, fork length, body mass, and Fulton’s K can be attributed to variability in carbon sourcing resulting from the large spatial footprint of our study area. In addition to relating these findings to Chinook salmon ecology, we offer guidance for future use of δ13C and δ15N analysis in studying early marine trophic interactions in anadromous fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The early marine phase is a period of extraordinary change for anadromous fish, such as juvenile Chinook salmon (Oncorhynchus tshawytscha). In addition to the physiological transformations associated with smoltification (McCormick et al. 2013), fish entering the marine environment are presented with new and potentially higher-quality trophic opportunities (e.g., piscivory; Litz et al. 2018). Consequent to these opportunities, juvenile Chinook enter a period characterized by unprecedented somatic growth and rapid increases in energy stores (MacFarlane 2010). These increases in growth and energy storage are sufficiently important to have been identified as primary influences on survival through the first marine year (Beamish and Mahnken 2001). Currently, mortality in the first marine year is thought to occur during two primary phases: a predation-based phase shortly after fish enter the ocean and a subsequent starvation-based phase in the fall and winter (Beamish and Mahnken 2001; Vega et al. 2016; Hertz et al. 2017; Beacham et al. 2018). Given the established relationships between scope for somatic growth, body condition (i.e., stored energy), and diet (Strand 2005; Zhou et al. 2009; Jorgensen et al. 2016); we expect size, provisioning, and consequent probability of surviving predation and starvation-based events to increase with the quality and/or quantity of dietary resources consumed during the period immediately prior to and following ocean entry (Litz et al. 2018). As such, characterizing the relationships between early trophic history, body size, and body condition represents a foundational element in our understanding of the mechanisms driving this early marine critical period (Beamish and Mahnken 2001) with associated implications for our understanding of Chinook salmon population ecology. Further, this relationship constitutes an important consideration for Chinook salmon management, particularly in areas with fluctuating or declining observed abundances, such as the eastern Bering Sea (Howard and von Biela 2023).
Previous examinations of the freshwater and early marine diets of Chinook and other Pacific salmon species have focused largely on dietary composition, using stomach content or stable isotope analysis to identify prey items to the lowest taxonomic level possible (Litz et al. 2017; Adams et al. 2017; Dale et al. 2017; Hertz et al. 2017; Chittenden et al. 2018). While many of these studies note intra- and inter-annual variability in dietary constitution based on prey availability, ocean conditions, and entry timing; few studies have focused on dietary variability among individuals and the potential relationships between inter-individual variability and differences in size, condition, and survival probability. While informative, these studies don’t allow for an examination of the relative importance of different influences on inter-individual variability in size and condition (e.g., early marine diet, freshwater diet, genetics, etc.), and, as a result, do not allow for targeted development of management and conservation strategies to address the most influential, and potentially sensitive, periods and processes in Chinook salmon life history.
Here, we report the results of research exploring potential relationships between inter-individual variability in diet and fork length, mass, and body condition in juvenile Chinook salmon from the southern Bering Sea using carbon (δ13C) and nitrogen (δ15N) stable isotope analysis of muscle and liver tissues. Previous research has shown that the proportions of δ13C in consumer tissue can be used to derive information on both the taxonomic breadth (e.g., insects/crustaceans/juvenile fish; Lorrain et al. 2011) and the geographic scope of feeding (e.g., freshwater/inshore/offshore; Radabaugh et al. 2013; Ruiz-Cooley and Gerrodette 2012; Lorrain et al. 2011; Cherel and Hobson 2007); while the proportions of δ15N in consumer tissues provides insight on trophic level (Jennings and van der Molen 2015; Post 2002). Further, because the C and N atoms in consumer tissues are entirely derived from dietary inputs (DeNiro and Epstein 1978, 1981), the proportions of δ13C and δ15N in consumer tissue are representative of assimilated dietary resources. By focusing on resources that have been assimilated, rather than merely ingested, analyses conducted using stable isotopes are: a) not affected by processes such as regurgitation during capture or differential digestion rates, and b) can provide information on diet composition for a longer period than other commonly used dietary characterization techniques, such as stomach content analysis. The exact temporal window of dietary inference allowed by δ13C and δ15N analysis is primarily driven by a tissue’s protein turnover rate (i.e., incorporation rate; Martinez del Rio et al. 2009 and references therein), and as such, can differ among species, individuals, and diets. A recent diet-switch experiment estimated that Chinook salmon skeletal muscle tissue equilibrates to the δ13C of a new diet in approximately 30 days (Bell-Tilcock et al. 2021). However, incorporation rates for δ15N in Chinook salmon muscle and δ13C and δ15N in Chinook salmon liver have yet to be derived, and comparative analyses of δ13C and δ15N tissue-specific incorporation rates within a single fish species are relatively rare. However, general characterizations of tissue-specific incorporation rates across vertebrates suggest that liver has faster δ13C and δ15N incorporation rates (i.e., represents more recent trophic interactions) than skeletal muscle (Martinez del Rio et al. 2009 and references therein). Despite being unable to assign specific temporal windows to liver stable isotope values, we sampled both skeletal muscle and liver to examine potential temporal variability or shifts in diet over time.
Methods
Field sample collection
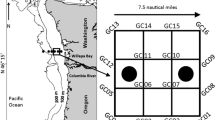
During August 10th–23rd of 2018 and 10th–29th of 2019, juvenile Chinook salmon were captured via surface trawling from the Alaska Department of Fish and Game research vessel R/V Pandalus using a Nordic 264 rope trawl (NET Systems Inc., Bainbridge Island, WA) modified to fish at the surface using buoys clipped to the headrope. Sampling occurred over a grid of 56 stations spaced 20 nautical miles (37.04 km) apart (Fig. 1) designed to cover the expected range of juvenile Chinook salmon nearshore habitat in the southern Bering Sea. Juvenile Chinook salmon caught during survey operations were recent emigrants from their natal freshwater systems into the nearshore marine environment. In 2018, a total of 39 stations were sampled due to inclement weather and a total of 50 stations were sampled in 2019 (Fig. 1). Immediately after collection, up to 10 juvenile Chinook salmon from each station were measured for fork length (mm), weighed (g) using a motion compensated scale, and frozen whole at −20 °C for stable isotope analysis. In addition, a caudal fin clip was removed from each sampled fish for genetic identification of river origin using the methods described in Howard et al. (2023). Briefly, genomic DNA was extracted using NucleoSpin 96 Tissue Kits (Macherey–Nagel; Düren, Germany). Samples were genotyped using Genotyping-in-Thousands by sequencing (GT-seq; Campbell et al. 2015). Quality control analyses included comparison of discrepancy rates between original genotypic data and genotypic data of 8% of samples that were re-extracted and re-genotyped. Individual genetic assignment analysis for stock-of-origin was performed using the R package rubias (Moran and Anderson 2019) and a new genetic baseline of 123 Chinook salmon populations developed specifically for western Alaska Chinook salmon (McKinney et al. 2019). Individual samples were assigned to one of five reporting groups: Asia, Yukon River, Coastal Western Alaska, Upper Kuskokwim River, and North Alaska Peninsula with >80% assignment probability. A total of 88 individual fish were sampled in 2018 and 2019 (46 in 2018 and 42 in 2019).
Juvenile Chinook salmon were collected from a grid of 56 stations spaced 20 nm apart designed to cover the expected range of nearshore habitat in the southern Bering Sea. In 2018 (left panel), a total of 39 stations were sampled with no set pattern. In 2019 (right panel), a total of 50 stations were sampled, but sampling was informed by 2018 catches, and stations closer to shore were prioritized. Closed circles indicate stations at which sampling was conducted, numeric labels denote the order in which stations were sampled, and X’s indicate stations at which no sampling occurred.
Stable isotope and statistical analyses
Whole fish were thawed and lateral skeletal muscle (2018 and 2019) and liver (2019) samples were removed for stable isotope analysis. Tissue samples were oven dried at approximately 42 °C, and ground to a fine, homogenous powder using a mortar and pestle. Approximately 1 mg of each homogenized sample was loaded into an individual tin capsule for stable isotope analysis. Tissue samples were analyzed for δ13C, δ15N, and percent C and N concentrations (hereafter [C] and [N], respectively) at the University of Wyoming Stable Isotope Facility using a Carlo Erba 1110 Elemental Analyzer (Carlo Erba Reagents, CE Instruments, ThermoQuest Italia S.p.A. Milan, Italy) coupled to a Thermo Delta Plus XP IRMS (Thermo Finnigan, Bremen, German). Long-term analyses of both quality control standards and reference materials run regularly throughout our sample analyses have yielded precisions of 0.3‰ for δ13C and 0.4‰ for δ15N. Results are presented using standard delta notation (δ13C, δ15N) in parts per million (‰) as δ = (Rsample/Rstandard − 1) * 1000, where Rsample and Rstandard are the ratios of the rare isotope to the abundant isotope (e.g., 13C/12C) of the sample and a standard reference material, in this case Vienna Pee Dee Belemnite for δ13C and atmospheric nitrogen for δ15N.
Potential effects of variable lipid content on the δ13C values of muscle and liver tissues (Sweeting et al. 2006) were arithmetically corrected for each sample using the model presented by Hoffman and Sutton (2010): δ13Cprotein = δ13Cbulk + (−6.39‰ * (3.76 − C:Nbulk))/C:Nbulk, where δ13Cbulk and C:Nbulk represent the uncorrected δ13C value and [C] to [N] ratio from our stable isotope analyses and −6.39 ‰ and 3.76 are mean estimates of Δδ13Clipid and C:Nprotein derived from a dataset of 48 mesopelagic and bathypelagic fish species (Hoffman and Sutton 2010). We chose to use these mean multispecies estimates because species-specific corrections from Chinook salmon have yet to be developed. δ15N values were not lipid corrected following Sweeting et al. (2006). All subsequent analyses were performed using the lipid-corrected δ13C values.
Body condition of individual fish was characterized using morphological attributes (i.e., fork length and body mass), Fulton’s K (calculated as K = W/L3 * 100,000, where W = body mass and L = fork length; Blackwell et al. 2000) and C:N as a proxy for lipid content(Fagen et al. 2011; Post et al. 2007). Potential relationships between δ13C, δ15N, and morphological and condition metrics were explored using Bayesian interpretations of generalized linear models (GLMs) using the bayesglm function in R version 4.1.3 (R core team 2022). Model covariates included year (for muscle), station, genetically-determined origin, C:N, percent difference from annual mean fork length, percent difference from annual mean mass, percent difference from annual mean Fulton’s K, and relevant interactions with δ13C and δ15N of muscle and liver acting as dependent variables. Interactions between covariates that we expected to be highly correlated (i.e., percent difference from annual mean fork length, percent difference from annual mean mass, and percent difference from annual mean Fulton’s K) were not included. All models used non-informative normal priors. Posterior distributions for credibility intervals were created using the MCMCregress function with MCMC chains each containing 10,000 samples with the first 3,000 removed. Covariates were determined to be influential if the 95% credibility interval did not contain zero (Hespanhol et al. 2018). In instances where interactions were detected, repeat model runs were conducted using the same methods but without including interactions. A second set of models, in which we explored isotopic covariation within and between muscle and liver tissues were also completed using the methods described above but with 2019 data only to facilitate comparison between muscle and liver tissues.
Results
Genetic analyses showed that of the 88 fish sampled, 76 were of coastal western Alaskan origin, one originated in the Upper Yukon River, one in the Upper Kuskokwim River, and 10 were either unassignable or were subject to failed genotyping (Howard et al. 2023). Because the overwhelming majority of the fish sampled were from the same region of origin, genetically determined origin was not included as a covariate in subsequent models.
Pooled bulk muscle tissue δ13C values ranged between −22.0 and −17.9‰ (−21.1 to −19.2‰ in 2018 and −22.0 to −17.9‰ in 2019; Table S1). Liver tissue (2019 only) bulk δ13C values ranged between −21.7 and −19.0‰ (Table S1). Arithmetic lipid corrections resulted in an average (± sd) change of −1.06 ± 0.11 ‰ in muscle δ13C values and 0.67 ± 0.38 ‰ in liver δ13C values (Fig. 2). Lipid corrected muscle tissue δ13C values ranged from −22.1 to −20.4 for 2018 and −23.1 to −19.0 for 2019 (Figs. 2 and 3). Lipid corrected liver tissue (2019 only) δ13C values ranged from −21.1 to −18.4 (Figs. 2 and 3). Pooled muscle tissue δ15N values ranged between 15.5 and 17.3‰ (16.2 to 17.2‰ in 2018 and 15.5 to 17.3‰ in 2019; Table S1 and Fig. 3). Liver tissue (2019 only) δ15N values ranged between 14.4 and 17.1‰ (Table S1 and Fig. 3).
Morphological metrics were slightly more variable in 2019, and condition metrics were similar between the 2 years (Fig. 4). Within years, fork length, mass, and Fulton’s K were relatively similar among individuals and ranged from 121 to 231 mm, 26 to 150 g, and 0.99 to 6.21, respectively in 2018 and from 141 to 264 mm, 36 to 238 g, and 1.04 to 1.51 in 2019. C:N values for muscle tissue ranged from 3.2 to 3.4 in 2018 and 3.2 to 3.3 in 2019. C:N values for liver ranged from 3.8 to 5.4.
95% credibility intervals of posterior distributions conducted on pooled muscle tissue samples identified year as an influential covariate for both δ13C and δ15N. No interactions were detected for either isotope (Table S2). Consequently, we ran a separate model for each year/isotope combination. When separated by year, none of the included covariates were influential predictors of δ13C or δ15N in muscle tissue in 2018 nor in muscle or liver in 2019 (Table S2). In addition, we did not detect any influential interactions in any of our models after separating by year. When run without potential interactions, 95% credibility intervals of posterior distributions indicated that none of included covariates were influential predictors of δ13C or δ15N in muscle tissue in 2018 or δ15N in muscle tissue in 2019 (Table 1). Percent difference from annual mean fork length, percent difference from annual mean mass, and percent difference from annual mean Fulton’s K were identified as influential predictors of muscle and liver δ13C in 2019. Sampling station was also identified as modestly influential (i.e., narrow 95% credibility interval positioned near 0) for liver δ13C. C:N ratio was the sole influential covariate identified for liver δ15N (Table 1). 95% credibility intervals of posterior distributions indicated that stable isotope values covaried among tissues (e.g., δ13C in liver and muscle) but not within tissues (e.g., δ13C and δ15N in liver; Table 2).
Discussion
Previous research has identified 1 and 3‰ as mean trophic discrimination values for δ13C and δ15N, respectively (Post 2002), indicating substantial variability in carbon source (i.e., taxonomic or geographic variability in prey) and trophic fractionation (i.e., feeding on different trophic levels). When interpreted using these guides, our results suggest taxonomic or geographic variability in feeding among individuals within the study period, with substantially less variability observed in 2018 than 2019 (range in δ13C: 2018 muscle = 1.7‰, 2019 muscle = 4.1‰, 2019 liver = 2.7‰). Conversely, we observed relatively little variability (i.e., ≤3‰) in the trophic levels of assimilated prey among individuals throughout the study period (range in δ15N: 2018 muscle = 1.0‰, 2019 muscle = 1.8‰, 2019 liver = 2.7‰). In addition, our results show that the relationships between diet (as indicated by δ13C and δ15N) and morphology/body condition vary by year, tissue type, and isotope. More specifically we found (1) no relationship between either isotope and morphology/body condition in 2018, (2) more enriched δ13C values associated with below average fork length and Fulton’s K and above average body mass in both muscle and liver tissues in 2019, and (3) more enriched δ15N values associated with higher C:N values for liver in 2019. In the following sections, we discuss the relevance of our results to juvenile Chinook salmon ecology and lessons for future studies employing stable isotope analysis to study early marine diets in Chinook salmon and other anadromous fish.
Similarities in diet, morphology, and body condition among individual juvenile Chinook salmon
Previous studies have highlighted the importance of the period between spawning and the first summer at sea in shaping Chinook salmon population dynamics in this region (Howard and von Biela 2023). Compared to more southerly Chinook salmon stocks in the contiguous United States, Chinook salmon in our study region have a long freshwater rearing period that includes a protracted downstream migration with limited spatial variability in prey types (Miller et al. 2020; Hertz et al. 2015). As a result of this comparatively longer freshwater period, juvenile Chinook salmon in our study region have the opportunity to grow larger and engage in consistent high-trophic level feeding (i.e., piscivory) while still in the freshwater environment compared to their more southerly conspecifics. Indeed, in both 2018 and 2019, muscle δ15N values for the juvenile Chinook salmon sampled in this study were similar (a) among individuals, and b) to those previously documented for adult Chinook salmon from the same geographic region (Satterfield IV and Finney 2002), suggesting widespread piscivory among late freshwater/early marine juvenile Chinook salmon in our study area. Further, the relative homogeneity in size and body condition observed in our results, coupled with the absence of clear relationships between those metrics and muscle δ15N values, suggests that Chinook salmon attain similar sizes due to increased time spent in the freshwater environment with consistent access to high-quality prey.
Liver δ15N values were similar (i.e., ≤3‰) to muscle δ15N values, further indicating that feeding on high trophic level prey is both widespread among our study organisms and temporally consistent. As previously mentioned, isotopic incorporation rates for 15N are unknown for both muscle and liver in Chinook salmon, and general characterizations of tissue-specific incorporation rates across vertebrates suggest that liver has faster δ13C and δ15N incorporation rates (i.e., represents more recent trophic interactions) than skeletal muscle (Martinez del Rio et al. 2009 and references therein). However, results from captive studies with two different teleosts show conflicting results. A study conducted on juvenile Atlantic croaker (Micropoganias undulatas) switched from high quality diets to medium or low-quality diet treatments (i.e., decreasing protein and lipid contents) found that the δ15N incorporation rate for liver was approximately 3 times faster than that for muscle, regardless of diet treatment (Mohan et al. 2016). In contrast, the relative difference in liver and muscle δ13C incorporation rates was highly variable among individuals and influenced by diet quality, specifically lipid content. Liver δ13C incorporation rates were between approximately 5 times faster and 1.6 times slower than δ13C incorporation rate for muscle tissues in low and medium quality diet treatments, respectively (Mohan et al. 2016). The authors attribute the difference in liver δ13C incorporation rates between diet treatments to increased lipid retention and metabolism in fish exposed to the low-quality diet. A second study conducted on captive Nile tilapia (Oreochromis nilotichus) fed identical diets, but different ration sizes, found that liver δ13C incorporation rates were between 2 and 4 times faster than δ13C incorporation rates for muscle depending on ration size (Carleton and Martinez del Rio 2012). Given that high-quality dietary resources are readily accessible to, and consumed by, juvenile Chinook salmon in our study area (Miller et al. 2020), we adopt the conservative interpretations that liver tissue will demonstrate a faster incorporation rate than skeletal muscle, the liver δ13C and δ15N values of fish in our study represent more recently consumed prey items than muscle, and the similarity in δ15N values between muscle and liver tissues suggests consistency in the trophic level of prey used by our study organisms across time. This interpretation is further supported by the covariation in stable isotope values between tissues shown in our results. Had the fish in this study engaged in a recent diet switch or more temporally diverse dietary habits, we would not expect the level of congruency in cross-tissue stable isotope values we observed. We note that the lack of covariation between the two isotopes observed within tissues is not unique to our study, and similar findings have been previously documented in a variety of taxonomic groups (e.g., deVries et al. 2015; Stanek et al. 2019).
When compared using 1 and 3‰ as mean trophic discrimination values for δ13C and δ15N (Post 2002), lipid corrected values for δ13C showed higher variability within and among years and between tissues than δ15N values. Inter-individual variability in δ13C values within years likely reflects spatial variability in resource use. While genetic analyses indicated that the majority of fish sampled for this study originated in Coastal Western Alaska, this region encompasses vast swaths of coastline, numerous spawning waterbodies, and hundreds of kilometers of migratory habitat (McKinney et al. 2019). As such, juvenile Chinook salmon from this region can demonstrate variability in individual δ13C values based solely on a broad range of available carbon sources and spatial variations in δ13C at the base of the food web (Satterfield and Finney 2002). Hertz et al. (2017) observed similar variability in δ13C values in juvenile Chinook salmon from the west coast of Vancouver Island. While the range of δ13C values presented by Hertz et al. (2017) was smaller than those observed here, the geographic region from which the salmon in our study originated is larger than Vancouver Island, with the potential for longer migration distances and greater spatial variability in δ13C. Further, prey sampling indicates a broader range of prey types, specifically juvenile fish, in our study area (Miller et al. 2020; Hertz et al. 2017, 2015), likely due to the larger geographic region. This broad geographic range in carbon sources may, in addition to temporal variations in δ13C at the base of the food web (Satterfield and Finney 2002), have also contributed to the inter-annual differences in the δ13C values we observed as a result of variations in the proportional representation of local assemblages of individuals from within different areas of the Coastal Western Alaska region between years. Unfortunately, without more spatially refined and spatially explicit sampling methods and the inability to identify river-of-origin to the Chinook salmon sampled, we are unable to assess the influence of spatial variations in δ13C on our results.
Concurrent local variations in morphology and δ13C resulting from the large spatial area, and consequent isotopic variability in available carbon sources, represented by the Coastal Western Alaska genetic assignment may also have resulted in the relationships between 2019 muscle and liver δ13C values and fork length, body mass, and Fulton’s K shown by our model results. We note; however, that the three relationships (i.e., δ13C and fork length, δ13C and body mass, and δ13C and Fulton’s K) are likely operating more or less independently on different aggregations of fish (rather than as concomitant products of the same mechanism) as it is arithmetically impossible for the same fish to have below average fork lengths, above average body masses, and below average Fulton’s K values. Further, the influence of sampling station on liver δ13C revealed by our model results provides the first evidence of the relative rates of isotopic incorporation in Chinook salmon liver and muscle, demonstrating that δ13C (a) incorporates more quickly in liver than muscle, and (b) liver provides a more recent snapshot of diet than muscle. Previous studies have noted differences of up to ~15 ‰ in the δ13C values of fish tissues feeding in freshwater and marine environments (Fuller et al. 2012). We did not observe differences approaching this magnitude in the δ13C values between tissues in our study, thereby precluding the conclusion that liver δ13C is entirely, or in large part, representative of marine diet. However, the influence of sampling station on liver δ13C values suggests that liver δ13C represents diet during at least some, albeit likely short, portion of the early marine phase in our study fish.
Unlike relationships between tissue δ13C values and morphological/condition metrics, the relationship between liver δ15N and C:N ratio revealed by our model results lends itself to a more straightforward interpretation. High trophic level prey, such as fish, is often also lipid rich. Further, a lipid rich diet would allow for increased consumer body fat reserves and an increased C:N ratio. Consequently, juvenile Chinook salmon engaged in high trophic level feeding, such as piscivory, would be expected to have comparatively high tissue δ15N values and C:N ratios. Because we observe this relationship in liver tissues but not muscle tissues, we must conclude that the relevant dietary resource is a relatively recent addition to the diets of our study organisms, potentially newly encountered marine prey. We do; however, advise a conservative perspective when interpreting the liver C:N results from our study as they are substantially lower than those reported by other authors. For example, Sweeting et al. (2006) found mean liver C:N values for captive young-of-the year European sea bass (Dicentrachus labrax) that were four to five times higher than the values observed in the current study. We attribute these observed disparities to differences in the energetic demands between study species and conditions. Given the liver’s large role as a storage site for lipids in fish (Sheridan 1988), the comparatively low C:N ratios in our study organisms may reflect low liver lipid levels resulting from (a) energy depletions associated with out migration, and/or (b) prioritization of energy for growth at the expense of creating endogenous energy reserves. Neither of these energy demands would be expected in a non-anadromous species fed to satiation in captive conditions (Sweeting et al. 2006). The C:N values for muscle observed in the current study were similar to those reported for other teleosts (Fagen et al. 2011; Post et al. 2007), including Atlantic salmon (Dempson et al. 2009).
Using stable isotopes to study the diets of juvenile Chinook salmon.
When conducting dietary analysis using stable isotopes, it is imperative that the temporal window(s) of inference provided by the sample tissue(s) match the phenomenon of interest. To accomplish this, tissue-specific isotopic incorporation rates must be determined using controlled experiments or relevant field data (He et al. 2021). In the case of this work, we were faced with extremely limited knowledge on the specific incorporation rates of tissue other than muscle, and as a result, we were initially unsure if liver stable isotope values would represent freshwater or early marine diets, a question that was, in part, answered by our results. By developing experimentally derived incorporation rates for a variety of juvenile Chinook salmon tissues (e.g., blood plasma, red blood cells, liver, smooth muscle, etc.), we could reliably interpret information on the dietary habits of juvenile Chinook salmon at a broader temporal scale, ranging from post-hatching to the early marine period. Further, by coupling these incorporation rates with specifically derived diet-to-tissue discrimination values, we can open the door to a reliable understanding of the roles played by specific prey sources in provisioning juvenile Chinook salmon at broad spatial and temporal scales. Given observed fluctuations in the numbers of returning adult Chinook salmon in many regions of Alaska (Riddell et al. 2022; ADF&G Chinook Salmon Research team 2013), and the potential future changes to juvenile Chinook salmon trophic ecology associated with global environmental change, understanding these important dietary relationships is imperative to successfully prepare for and potentially mitigate future challenges faced by these high-latitude stocks.
Data availability
The stable isotope data analyzed for this manuscript are available in Table S1. Other data generated and/or analyzed during the current study are not publicly available due to potential use in future manuscripts. These are available from the corresponding author on reasonable request.
References
Adams JN, Brodeur RD, Daly EA, Miller TW (2017) Prey availability and feeding ecology of juvenile Chinook (Oncorhynchus tshawytscha) and coho (O. kisutch) salmon in the northern California Current ecosystem, based on stomach content and stable isotope analyses. Mar Biol 164:98. https://doi.org/10.1007/s00227-017-3095-z
ADF&G Chinook Salmon Research Team (2013) Chinook Salmon Stock Assessment and Research Plan, 2013. Special Publication No. 13-01, Alaska Department of Fish and Game, Anchorage, AK
Beacham TD, Araujo HA, Tucker S, Trudel M (2018) Validity of inferring size-selective mortality and a critical size limit in Pacific salmon from scale circulus spacing. PLoS ONE. https://doi.org/10.1371/journal/pone.0199418
Beamish RJ, Mahnken C (2001) A critical size and period hypothesis to explain natural regulation of salmon abundance and the linkage to climate and climate change. Prog Oceanogr 49:423–437
Bell-Tilcock M, Jeffres CA, Rypel AL, Willmes M, Armstrong RA, Holden P, Moyle PB, Fangue NA, Katz JVE, Sommer TR, Conrad JL, Johnson RC (2021) Biogeochemical processes create distinct isotopic fingerprints to track floodplain rearing of juvenile salmon. PLoS ONE. https://doi.org/10.1371/journal.pone.0257444
Blackwell BG, Brown ML, Willis DW (2000) Relative weight (Wr) status and current use in fisheries assessment and management. Rev Fish Sci 8(1):1–44
Campbell NR, Harmon SA, Narum SR (2015) Genotyping-in-Thousands by sequencing (GT-seq): a cost effective SNP genotyping method based on custom amplicon sequencing. Mol Ecol Resour 15(4):855–867. https://doi.org/10.1111/1755-0998.12357
Carleton SA, Martinez del Rio C (2012) Growth and catabolism in isotopic incorporation: a new formulation and experimental data. Funct Ecol 24:805–812
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287
Chittenden CM, Sweeting R, Neville CM, Young K, Galbraith M, Carmack E, Vagle S, Dempsey M, Eert J, Beamish RJ (2018) Estuarine and marine diets of out-migrating Chinook Salmon smolts in relation to local zooplankton populations, including harmful blooms. Estuar Coast Shelf Sci 200:335–348
Dale KE, Daly EA, Brodeur RD (2017) Interannual variability in the feeding and condition of subyearling Chinook salmon off Oregon and Washington in relation to fluctuating ocean conditions. Fish Oceanogr 26(1):1–16
Dempson JB, Braithwaite VA, Doherty D, Power M (2009) Stable isotope analysis of marine feeding signatures of Atlantic salmon in the north Atlantic. ICES J Mar Sci 67:52–61
Deniro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochemica Et Cosmochimica Acta 42:495–506
Deniro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochemica Et Cosmochimica Acta 45:341–351
deVries MS, Martinez del Rio C, Tunstall TS, Dawson TE (2015) Isotopic incorporation rates and discrimination factors in mantis shrimp crustaceans. PLoS ONE 10(4):e0122334
Fagen KA, Koops MA, Arts MT, Power M (2011) Assessing the utility of C: N ratios for predicting lipid content in fishes. Can J Fish Aquatic Sci 68:374–385. https://doi.org/10.1139/F10-119
Fuller B, Müldner G, Van Neer W, Ervynck, Richards MP (2012) Carbon and nitrogen stable isotope analysis of freshwater, brackish, and marine fish from Belgan archeological sites (1st and 2nd millennium AD). J Anal At Spectrom. https://doi.org/10.1039/C2JA10366D
He M, Liu F, Wang F (2021) Determination of the stable isotope discrimination factor if wild organisms: a case study of the red swamp crayfish in integrated rice-crayfish (Procambarus clarkia) culture without artificial diets. Isot Environ Health Stud. https://doi.org/10.1080/10256016.2021.2008380
Hertz E, Trudel M, Brodeur RD, Daly EA, Eisner L, Farley EV Jr, Harding JA, MacFarlane RB, Mazumder S, Moss JH, Murphy JM, Mazumder A (2015) Continental-scale variability in the feeding ecology of juvenile Chinook salmon along the coastal Northeastern Pacific Ocean. Mar Ecol Prog Ser. https://doi.org/10.3354/meps11440
Hertz E, Trudel M, Tucker S, Beacham TD, Mazumder A (2017) Overwinter shifts in the feeding ecology of juvenile Chinook salmon. ICES J Mar Sci 74:226–233
Hespanhol L, Vallio CS, Costa LM, Saragiotto BT (2018) Understanding and interpreting confidence and credible intervals around effect estimates. Braz J Phys Ther. https://doi.org/10.1016/bjpt.2018.12.006
Hoffman JC, Sutton TT (2010) Lipid correction for carbon stable isotope analysis of deep-sea fishes. Deep-Sea Research I. https://doi.org/10.1016/j.dsr.2010.05.003
Howard K, Garcia S, Liller Z, Harris B, Wolf N (2023) Final report for the southern Bering sea juvenile chinook salmon survey. Saltonstall-Kennedy Project NA17NMF4270241. Prepared July 2023
Howard KG, von Biela V (2023) Adult spawners: a critical period for subarctic Chinook salmon in a changing climate. Glob Change Biol. https://doi.org/10.1111/gcb.16610
Jennings S, van der Molen J (2015) Trophic levels of marine consumers from nitrogen stable isotope analysis: estimation and uncertainty. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsv120
Jorgensen C, Enberg K, Mangel M (2016) Modelling and interpreting fish bioenergetics: a role for behavior, life-history traits and survival trade-offs. J Fish Biol 88:389–402
Litz MNC, Miller JA, Copeman LA, Teel DJ, Weitkamp LA, Daly EA, Claiborne AM (2017) Ontogenetic shifts in the diets of juvenile Chinook Salmon: new insight from stable isotopes and fatty acids. Environ Biol Fish. https://doi.org/10.1007/S10641-016-0542-5
Litz MNC, Miller JA, Brodeur RD, Daly EA, Weitkamp LA, Hansen AG, Claiborne AM (2018) Energy dynamics of subyearling Chinook salmon reveal the importance of piscivory to short-term growth during early marine residence. Fish Oceanogr. https://doi.org/10.1111/fog.12407
Lorrain A, Arguelles J, Alegre A, Betrand A, Munaron J-M, Richard P, Cherel Y (2011) Sequential isotopic signature along gladius highlights contrasted individual foraging strategies of Jumbo Squid (Dosidicus gigas). PLoS ONE 6(7):e22194. https://doi.org/10.1371/JOURNAL.PONE.022194
MacFarlane RB (2010) Energy dynamics and growth of Chinook salmon (Oncorhynchus tshawytscha) from the Central Valley of California during the estuarine phase and first ocean year. Can J Fish Aquat Sci. https://doi.org/10.1139/F10-080
Martínez del Rio C, Wolf N, Carleton SA, Gannes LZ (2009) Isotopic ecology ten years after a call for more laboratory experiments. Biol Rev 84:91–111
McCormick SD, Sheehan TF, Björnsson BT, Lipsky C, Kocik JF, Regish AM, O’Dea MF (2013) Physiological and endocrine changes in Atlantic salmon smolts during hatchery rearing, downstream migration, and ocean entry. Can J Fish Aquat Sci. https://doi.org/10.1139/cjfas-2012-0151
McKinney GJ, Pascal CE, Templin WD, Gilk-Baumer SE, Dann TH, Seeb LW, Seeb JE (2019) Dense SNP panlems resolve closely related Chinook salmon populations. Can J Fish Aquat Sci. https://doi.org/10.1139/cjfas-2019-0067
Miller K, Shaftel D, Bogan D (2020) Diets and prey items of juvenile Chinook (Oncorhynchus tshawytscha) and coho salmon (O. kisutch) on the Yukon Delta. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-AFSC-410, p 54
Mohan JA, Smith SD, Connelly TL, Attwood ET, McClelland JW, Herza SZ, Walther BD (2016) Tissue-specific isotope turnover and discrimination factors are affected by diet quality and lipid content in an omnivorous consumer. J Exp Mar Biol Ecol 479:35–45. https://doi.org/10.1016/j.jembe.2016.03.002
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG (2007) Getting to the fat of the matter: models, methods, and assumptions for dealing with lipids in stable isotope analyses. Oecologia. https://doi.org/10.1007/s00442-006-0630-x
R core team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.prg/
Radabaugh KR, Hollander DJ, Peebles EB (2013) Seasonal δ13C and δ15N isoscapes of fish populations along a continental shelf trophic gradient. Cont Shelf Res 68:112–122
Riddell BE, Howard KG, Munro AR (2022) Salmon returns in the northeast pacific in relation to expedition observations (and next steps). N Pac Anadromous Fish Comm Techn Rep 18:115–139
Ruiz-Cooley RI, Gerrodette T (2012) Tracking large-scale latitudinal patterns δ13C and δ15N along the E. Pacific using epi-mesopelagic squid as indicators. Ecosphere. https://doi.org/10.1890/ES12-00094.1
Satterfield FR IV, Finney BP (2002) Stable isotope analysis of Pacific salmon: insight into trophic status and oceanographic conditions over the last 30 years. Prog Oceanogr 53:231–246
Sheridan MA (1988) Lipid dynamics in fish: aspects of absorption, transportation, deposition, and mobilization. Comp Biochem Physiol B Comp Biochem 90:679–690
Stanek AE, Wolf N, Welker JM, Jensen S (2019) Experimentally-derived incorporation rates and diet-to-tissue discrimination values for carbon and nitrogen stable isotopes in gray wolves (Canis lupus) fed a marine diet. Can J Zool. https://doi.org/10.1139/CJZ-2019-0049
Strand Å (2005) Growth and bioenergetic models and their application in aquaculture of perch (Perca fluviatilis). PhD thesis. Sveriges Lantbruks Universitet, Vattenbruksinstitutionen, Umeå
Sweeting CJ, Polunin NVC, Jennings S (2006) Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid Commun Mass Spectrom 20:595–601. https://doi.org/10.1002/rcm.2347
Vega SL, Sutton TM, Murphy JM (2016) Marine-entry timing and growth rates of juvenile Chum Salmon in Alaskan waters of the Chukchi and northern Bering seas. Deep Sea Res II 135:137–144
Zhou Q, Xie P, Xu J, Ke Z, Guo L (2009) Growth and food availability of silver and bighead carps: evidence from stable isotope and gut content analysis. Aquacult Res 40:1616–1625
Acknowledgements
The authors wish to thank the crew of the R/V Pandalus, Ben Gray at ADF&G, and the team at the University of Wyoming Stable Isotope Facility. This publication was prepared by the authors using Federal funds under Award NA17NMF4270241 from NOAA Fisheries, U.S. Department of Commerce. The statements, findings, conclusions, and recommendations are those of the author(s) and do not necessarily reflect the views of NOAA Fisheries or the U.S. Department of Commerce.
Funding
This publication was prepared by the authors using Federal funds under Award NA17NMF4270241 from NOAA Fisheries, U.S. Department of Commerce. The statements, findings, conclusions, and recommendations are those of the author(s) and do not necessarily reflect the views of NOAA Fisheries or the U.S. Department of Commerce.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analyses were performed by NW, SG, and KH. The first draft of the manuscript was written by NW and all authors commented on previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Capture and collection activities associated with this work were part of a broader survey program conducted under United States National Marine Fisheries Service Letters of Acknowledgement 2018–01 and 2019–03. All fish capture and slaughter 1) followed established protocol for survey and commercial-fishing activities, and 2) was conducted in consideration of the American Veterinary Medical Association (AVMA) guidelines for the humane slaughter of animals.
Consent to participate
NA.
Consent to publish
NA.
Additional information
Responsible Editor: V. Paiva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wolf, N., Garcia, S., Harris, B.P. et al. Stable isotopes, morphology, and body condition metrics suggest similarity in the trophic level and diversity in the carbon sources of freshwater and early marine diets of Chinook salmon. Mar Biol 171, 75 (2024). https://doi.org/10.1007/s00227-024-04392-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04392-8