Abstract
Coral reef fish species experience variable environments and may employ a range of reproductive strategies to help them maximize fitness. Here, we explore patterns of variation in size- and sex-dependent spawning strategies (pair spawning, group spawning, and streaking tactics) of a sex-changing, highly iteroparous fish (the sixbar wrasse Thalassoma hardwicke) across the lunar month, and in relation to proximity to the reef edge. We test the hypothesis that adults vary their investment strategies as a function of body size. We evaluated reproductive behaviour patterns at 2–3 days intervals over 5 consecutive months, and found that frequencies of pair spawning, group spawning, and streaking attempts all significantly increased around the new moon and were greatest in the most exposed territories (i.e., those located closest to the reef edge). Smaller females and IP males largely curtailed reproductive activities during the full moon and third quarter moon, while larger individuals reproduced more consistently across the lunar month. Size-dependent reproductive decisions appeared to mediate frequencies of specific reproductive tactics (e.g., prevalence of pair spawning, group spawning, and streaking strategies). We speculate that (1) optimal tactics are shaped by size- and sex-dependent trade-offs between reproduction and growth in relation to environmental conditions, and that (2) individual investment decisions determine the prevailing reproductive mode at a spawning site. Variation in female choice might thereby act as a mechanism for maintaining these two reproductive modes (pair spawning and group spawning) in a population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reef fishes exhibit an extraordinary diversity of reproductive tactics that vary both within and among species (Aldenhoven 1986; Johannes 1978; Warner and Hoffman 1980a, b; Warner 1984a; Shapiro 1991; Munday et al. 2006). Many coral reef fishes spawn with a lunar rhythm, suggesting that particular phases of the moon might enhance offspring fitness (Johannes 1978; Robertson et al. 1990; Claydon et al. 2014; Shima et al. 2020). For example, spawning at particular times of the lunar cycle may increase transport of fertilized eggs and larvae off the reef and away from higher concentrations of reef-associated predators (e.g., via tidal effects: Robertson et al. 1990; Claydon 2004; Claydon et al. 2014). Alternatively, the lunar cycle may simply provide a useful cue that allows some species to synchronize reproductive activities to maximize fertilization rates, and/or to minimize predation rates on eggs (i.e., via predator swamping; Lobel 1978; Colin and Clavijo 1988; Robertson et al. 1990; Domeier and Colin 1997; Nemeth et al. 2007). Spawning on a lunar rhythm may also enable offspring to return to the reef at an optimal time (e.g., Shima et al 2018), and/or to complete their pelagic larval development during times that maximize feeding opportunities and/or minimize predation risk (Shima and Swearer 2019; Shima et al. 2020, 2021).
Spawning patterns may additionally be shaped by perceived risks to parents (Warner 1998). This is particularly true for individuals that can spawn frequently, and over many reproductive seasons—where any single reproductive event contributes only marginally to lifetime fitness. In such cases, individuals should prioritize their own survival over that of their offspring. Such tactics may be particularly prevalent in species with protogynous hermaphroditism (i.e., sex change from female to male). For sequential protogynous hermaphrodites (i.e., maturing first to female, then to male), body size often determines status within the dominance hierarchy for both sexes (Munday et al. 2006). Lifetime fitness can be maximized for individuals that ascend the dominance hierarchy and become territorial males (because males in this terminal phase can monopolize mating opportunities with many females (Warner 1984a; Munoz and Warner 2003). Individuals of different sizes or social status may, therefore, adopt different reproductive strategies or at least weigh up risks differently.
Alternative reproductive tactics have been described for males of many sex-changing fish species (Henson and Warner 1997; Alonzo and Warner 2000). Smaller males with limited energetic resources or competitive ability often engage in alternative reproductive tactics that include “streaking” (i.e., parasitising a pair-spawning event), “sneaking” (i.e., surreptitious pair-spawning within another male’s territory), and “group spawning (i.e., spawning as part of a group of > 3 individuals; Table 1). In many cases, smaller males mimic phenotypes and behaviour patterns of females to gain access to another male’s territory (Taborsky et al. 1987; Warner and Hoffman 1980a, b), and they are often referred to as ‘initial phase’ (IP) males (because they resemble the initial female stage, and to differentiate them from territory-holding ‘terminal phase’ or ‘TP’ males). Maturation to an IP male may be a strategy to circumvent energetic costs associated with courtship or competitive interactions (i.e., necessary costs for a TP male), to potentially redirect a higher proportion of energetic reserves to growth (Hoffman 1983; Warner 1984b; Taborsky 1998). The efficacy of these alternative reproductive tactics may be frequency-dependent. For example, if IP males become locally common, TP males may be less able to defend their territories. Above a certain threshold density of IP males, the territorial strategy of TP males becomes ineffective to the point that most matings go to IP males in large group spawning assemblages (Warner and Hoffman 1980a, b; Warner and Robertson 1978).
Competition among females for access to males appears to be less common (or at least, less apparent) in protogynous hermaphrodites (Warner 1998; Taborsky and Brockmann 2010). However, females must make decisions about where, when, and with whom they spawn. Smaller females may be more vulnerable to predators during and immediately after spawning, and, therefore, they may be more selective about the location and timing of spawning (Robertson and Hoffman 1977; Warner 1998; Rideout et al. 2005; McBride et al. 2015). Larger females may allocate a larger proportion of their energetic reserves to current reproduction, and, therefore, they may spawn more consistently through time and choose spawning locations that promote offspring survival. Consequently, size-dependent variation in predation risk (Hixon and Beets 1993; DeMartini et al. 2005; Welsh et al. 2013) may lead to size-structured spatial and temporal variation in spawning activities. Small IP males might show the same level of reluctance in employing their sperm allocation strategies (Warner 1998), because they too have the potential to develop into highly successful territorial males.
In short, individuals may vary their reproductive tactics, as a function of both their body size and their sex in a hierarchical structured population. Moreover, the optimal tactic may further depend upon on environmental conditions. Females engage less risky behaviours when predators are present, and/or visibility is obscured by turbidity or light; this could favour group spawning and/or spawning in more protected locations away from the reef edge. If conditions for offspring survival vary on a lunar cycle (e.g., Shima et al. 2020, 2021), then risk-averse (e.g., smaller) females may limit reproduction to times with the greatest predicted payoff. This could drive patterns of lunar periodicity in reproductive behaviour patterns that vary with body size. Few studies have attempted to evaluate context-dependence in size-structured reproductive investment decisions.
Here, we evaluate variation in spawning strategies across the lunar cycle, and in relation to proximity to the reef edge for the sixbar wrasse (Thalassoma hardwicke). Specifically, we address the following questions: (1) Do frequencies of pair spawning and group spawning vary across the lunar month, and with location? (2) Do smaller females concentrate their activities around specific times of the lunar month and/or do they prefer specific locations for reproduction? (3) Do females have varying preferences in their choice of male phenotypes (territorial males versus IP males)? (4) Do males engaging in alternative reproductive tactics (i.e., streaking, sneaking, group spawning) vary in their success across a lunar month, and with location?
Methods
Study species
We focus on the sixbar wrasse, Thalassoma hardwicke—a small bodied reef fish that is common on shallow coral reefs across the Indo-Pacific. This species is a protogynous hermaphrodite, and highly iteroparous (Mitterwallner 2020). All individuals first develop as initial phase males or females, and some of these individuals will develop secondarily as brightly coloured ‘terminal phase’ males. Terminal phase males defend and attempt to control mating territories, but their success may depend upon local densities of smaller initial phase males, which vary in space and time (Mitterwallner 2020; Mitterwallner and Shima 2022). Previously, we found that overall spawning activity varies strongly with the lunar cycle (with the most spawning activity occurring during the new moon) and with proximity to the reef edge (where advection of eggs—and also predation risk to adults—is presumed to be greatest: Johannes 1978; Lobel 1978; Sancho et al. 2000).
Field surveys
We surveyed spawning patterns of the sixbars from February to June 2017, at two focal sites on the north shore of Mo’orea, French Polynesia. These two focal sites (hereafter, S1 and S2) were situated at the reef edge, near passes, on the north-eastern side of the island (Fig. 1). Within each site, we identified and mapped three focal territories (T1, T2, T3) of different terminal phase (TP) males. These territories were distributed along a gradient from “exposed” (i.e., T1 directly next to the reef edge) to “sheltered” conditions (i.e., T3 about 20 m away from the reef edge; Fig. 1).
Locations of two spawning sites (S1 and S2) within a lagoon on the north shore of Mo’orea, French Polynesia (a, b). Within each site, we monitored focal territories (T1, T2, T3) of terminal phase males (c). These territories were distributed along a gradient from exposed (i.e., T1 closest to the reef edge) to sheltered conditions (i.e., T3 furthest from the reef edge)
We surveyed these focal territories in each site on alternate days. Each site was surveyed between 14 and 16 h, ~ 3–5 times per week over five lunar cycles (i.e., 20 weeks). A preliminary study indicated that spawning activity was consistently maximal and best estimated by this observational timeframe (Mitterwallner 2020). During each survey, a single observer (PM) recorded spawning activities within each focal TP territory (n = 3) over a 20 min duration. We recorded frequencies of the following behaviour patterns associated with reproduction: (1) pair spawning, (2) group spawning, (3) streaking attempts (non-territorial males attempting to enter territories, but chased away by the territory holder), (4) successful streaking (streaker males successfully engaged in spawning), and (5) sneaking events (for more detailed descriptions of spawning behaviours, see ethogram in Table 1). We estimated the body sizes (total lengths, TL) and phenotypes [initial phase (IP) or terminal phase (TP)] of all individuals involved in a behaviour pattern. Visual size estimates were continuously calibrated against objects of known size (i.e., PVC pipe, cut to length, and suspended horizontally in the water column near areas of fish activity) to increase consistency and accuracy of visual estimates. We note that it is was not possible to differentiate between sexes when estimating sizes in a group spawning rush, because IP males and females are phenotypically indistinguishable from another (although a TP chasing an IP might enable us to infer the IP’s sex as male). Additionally, the nature of group spawning events (which occurred rapidly, and included up to 30 individuals) facilitated only a coarse estimate of size distribution for a collective group. We also note that our decision to sample reproductive activity within territories of focal TPs is likely to bias relative frequencies of alternative tactics in favour of pair-spawning with TP males.
Data analysis
Overview of approach
We evaluate variation in (1) frequencies of spawning events and (2) body sizes of individuals engaging in reproductive strategies. We explore patterns of variation with respect to the lunar cycle and across a spatial gradient of exposure. We used a periodic regression (linear–circular regression) approach to model the cyclical nature of the lunar cycle. Following methods of deBruyn and Meeuwig (2001), the calendar date of each observation was converted to a ‘lunar day’ (0–29.5; day 0 corresponds to the new moon, day 15 to the full moon, etc.). Lunar days were then divided into 360° (or 2π radians) to allocate each day an angular equivalent, theta (θ). The cyclical pattern of the lunar calendar was then expressed by sine and cosine transformations of theta. The cosine term describes a phase shift near 0° or 180° (full moon and new moon), and the sine term describes a phase shift between 90° and 270° (first and last quarter). A positive cosθ coefficient reflects a peak in activity at the new moon, and a negative cosθ coefficient reflects a peak in activity at the full moon. A significant positive sinθ coefficient corresponds to a peak in activity around the first quarter moon, and a significant negative sinθ coefficient corresponds to a peak in activity around the last quarter moon. The transformed data can then be analysed using simple linear or linear mixed effect regression. Specifically, we modelled dependent variables (detailed below) as a function of a sinθ and cosθ term (to capture the cyclical components of the first/last quarter moons and new/full moons, respectively), and spatial terms (territory position and site; to capture gradients in exposure, etc.).
Variation in pair spawning and group spawning strategies
We evaluated patterns and sources of variation in frequencies of (1) pair spawning, and (2) group spawning. Because our observations consisted of counts, we used an underlying Poisson error distribution. We modelled fixed effects of the lunar cycle (sinθ, cosθ), site and territory position. We included date as a random effect to account for non-independence of observations made on the same dates (GLMER package). Pair spawning and group spawning were evaluated in separate models, and in each case the full model took the following form:
Variation in sizes of spawners
We evaluated patterns and sources of variation in the body sizes (TL) of initial phase fish engaged in (1) pair spawning, and (2) group spawning. Here, we used an underlying Gaussian error distribution to reflect the more continuous nature of our size estimates. We modelled fixed effects of the lunar cycle (sinθ, cosθ), site and territory position. We included a random effect of date in preliminary tests, but this did not contribute importantly to our models evaluating size, so we excluded it in final reported analyses. Sizes of fish engaging in pair spawning and group spawning were evaluated in separate models, and in each case the full model took the following form:
We modelled site as a fixed factor because we wished to evaluate the influence of site-specific characteristics on reproductive investment decisions. For all models described above, we did not include interaction terms because our preliminary analyses indicated that their inclusion resulted in only marginal improvements of model fits. Inspection of normalized quantile-plots indicated a satisfactory fit to the data for all models. All analyses were performed with the lme4 package (Bates et al. 2013) in R (R Core Development Team 2019).
To facilitate visualization of model parameters, we calculated the predicted response of the dependent variables to the lunar cycle with the ‘predict’ function of the lme4 package. For the effect of categorial variables on spawning patterns, we estimated marginal means for each predictor, with covariates held at their mean value (using function emmeans in the R package emmeans; Lenth 2018). The relative importance of predictors in the best-fit model was assessed by partitioning R2 (relative variance contribution) with the “relaimpo” R package (Grömping 2006), which utilizes the Lindeman, Merenda and Gold method (Lindeman et al. 1980).
Evaluating female preferences for male phenotypes (TP versus IP males)
To further explore patterns of variation in female engagement with male phenotypes (pair spawning TP males versus group spawning IP males), we conducted a set of quantile regressions. Specifically, we evaluated correlations between (1) pair spawning frequency (pair spawns × territory−1 × day−1) and corresponding group spawning frequency (group spawns × territory−1 × day−1); and (2) body sizes of pair spawning individuals (mean TL × territory−1 × day−1) and group spawning frequency (group spawns × territory−1 × day−1). A negative correlation between pair spawning and group spawning frequencies is consistent with females exhibiting alternative tactics. A correlation between body size and spawning frequency would indicate disproportionate participation by individuals of particular sizes. We used quantile regression because preliminary analyses suggested ‘wedge-shaped’ patterns in these relationships. Quantile regressions enabled us to evaluate whether relationships were stronger for the upper boundaries. Wedge shaped distributions in a dataset imply that there may be only a weak or no relationship between the mean of the response variable distribution and the measured predictor variable, but a potentially stronger relationship at the upper boundaries of data distributions. Therefore, we described rates of change in quantiles at maximal response (95% quantiles), in comparison to quantiles at medium response (50% quantiles) with the quantreg package in R (Koenker 2011). To supplement these analyses and facilitate further inferences, we conducted simple linear regressions between densities of sixbars at the spawning site and (1) pair spawning frequency; and (2) group spawning frequency; we used the lme4 package in R (Bates et al. 2013).
Variation in male streaking strategies
Additionally, we used Eqs. 1 and 2 to evaluate patterns and sources of variation in male streaking attempts (frequency, and size of involved males trying to enter territories but being chased away) and successful streaking events (frequency).
Results
We observed 649 bouts of spawning over 71 days of observation within the focal territories. 59% of these were pair spawning, 37% were group spawning, 3.5% were streak spawning, and 0.7% were sneak spawning.
Variation in pair spawning and group spawning strategies
Pair spawning frequency was strongly influenced by the cosθ term describing moon phase. This parameter predicts highest spawning frequencies close to the new moon (frequency of spawning on new moon increases by ~ 80% relative to full moon (GLMER, estimate ± SE = 0.54 ± 0.17, p < 0.001 marg. r2 = 22.8). Spawning frequency remained low from the first quarter to the full moon (Fig. 2a). Group spawning frequency was also strongly influenced by the cosθ term (GLMER, estimate ± SE = 2.03 ± 0.50, p < 0.001, marg. r2 = 33.9; Fig. 2a), but spawning frequencies ceased entirely at the full moon and then rapidly increased (~ sixfold) in the lead up to the new moon (Fig. 2a, Table 2).
Main sources of variation in spawning frequency (upper row) and size of spawning females (lower row). a Smoothed curve (± 95% confidence interval) of the lunar effect on predicted pair and group spawning frequency during the 29.5 day lunar cycle. b Variation in spawning frequency among territories (Territory 1 is closest to reef edge, Territory 3 furthest from reef edge). c Smoothed curve (± 95% confidence interval) of the lunar effect on predicted size of pair spawning females and group spawning individuals (females and IP males) during the 29.5 day lunar cycle. d Variation in sizes of pair spawning females and group spawning individuals (females and IP males) among territories. Panel b and d give marginal means back-transformed to the original scale (+ SE) estimated with other fixed effects held at their mean values
Pair spawning and group spawning frequencies markedly differed across territories that varied in their proximity to the reef edge. Territories located closest to the reef edge had greater frequencies of pair and group spawning relative to territories located further from the reef edge, and this disparity was most pronounced for pair spawning (Territory 3: GLMER, estimate ± SE = − 2.11 ± 0.19, p < 0.001 marg. r2 = 64.1; Fig. 2b and Table 2). Group spawning frequencies were ~ 90% higher at site S2, an area at the extreme down-current edge of the reef (GLMER, estimate ± SE = 3.43 ± 0.80, p < 0.001, marg. r2 = 54.3; Table 2). In contrast, pair spawning frequencies were significantly higher at site S1, an area located further away from the channel entrance (GLMER, estimate ± SE = − 0.78 ± 0.24, p < 0.001, marg. r2 = 13.0, Table 2).
Variation in sizes of spawners
Females of a wide range of sizes engaged in both pair spawning and group spawning. Pair spawning females ranged from 7 to 17 cm TL (mean ± 1SE: 12.1 ± 1.3 cm). Group spawning rushes consisted of 5–30 recorded individuals, with sizes varying from 9 to 16 cm (12.6 ± 1.5 cm). Sizes of the six focal territory owners ranged between 18 and 20 cm.
Variation in sizes of female pair spawners was strongly influenced by the lunar cycle. The stronger influence of the sinθ component (GLM, estimate ± SE = − 0.3 ± 0.1, p < 0.001, marg. r2 = 13.6, Fig. 2c) indicates that the mean size of pair spawning females was greatest on the third quarter moon (potentially indicating that a disproportionate number of large females were choosing to spawn at this time, and/or small females were opting out; Fig. 2c). Mean size of female spawners was lowest just after the new moon (potentially indicating a greater number of smaller females and/or fewer larger females spawning at this phase of the moon; Fig. 2c).
The mean size of group spawners tended to be larger, but also more variable, relative to pair spawners. Mean size of fish engaging in group spawning did not vary significantly with the lunar cycle (Table 2), although the pattern of variation with respect to moon phase was qualitatively similar to that observed for pair spawning (Fig. 2c).
Though sizes of spawners varied with moon phase, spawning locations had a much greater effect on sizes of pair spawners (i.e., territory location accounted for 48% of the variance in pair spawner size). Mean sizes of females involved in pair spawning significantly decreased along a gradient from exposed to sheltered territories (an ~ 8% reduction in size from T1 to T3; GLM, Territory 3: estimate ± SE = − 0.96 ± 0.24, p < 0.001, marg. r2 = 48.6; Fig. 2d). The model also indicated, that average size of pair spawning females was significantly higher in a spawning site closer to the reef crest (S2) relative to a spawning site located more centrally within the lagoon (GLM, estimate ± SE = 0.63 ± 0.13, p < 0.001, marg. r2 = 28.0; Table 2). Sizes of group spawning individuals did not vary significantly among territories (Fig. 2d) or spawning site.
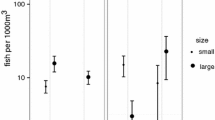
Because the patterns of variation in mean size of female pair spawners might be the result of small or large females opting in or opting out of spawning at different locations or time, we conducted an additional analysis to attempt to resolve size-dependent spawning patterns in our data. For this exploratory analysis, we categorized female pair spawners into two size classes: “small” individuals (7–11 cm) and “large” individuals (12–16 cm). We evaluated variation in pair spawning frequencies of small and large females relative to the lunar cycle (e.g., pair spawning frequency ~ size category* sinθ + size category* cosθ) with a GLM model (Poisson error distribution). Overall, spawning frequencies of small and large individuals were highest around new moon (as indicated by a significant positive cos term) and lowest around third quarter (Table 3). However, as indicated by the model, a significantly higher proportion of large individuals was engaged in spawning around third quarter moon, relative to smaller individuals (GLM, sinθ (large): estimate ± SE = − 0.40 ± 0.18, p = 0. 03; Fig. 3).
Evaluating female preferences for male phenotypes (TP versus IP males)
Quantile regression suggests that the frequency of pair spawning declines with increasing frequency of group spawning, particularly at the upper boundaries of the data distribution (Table 4). The 95% quantile regression indicated a significant negative relationship (p < 0.01) while the 50% regression line exhibited no detectable slope. A second quantile regression indicated that upper range of sizes (the 95% quantile) of female pair spawners increased with increasing group spawning frequency (Table 4). These patterns indicate that the upper extents of the data distribution for both pair spawning frequency and the sizes of fish engaging in pair spawning are sensitive to the frequency of group spawning activity (Table 4, Fig. 4a, b).
Quantile regressions (tau = 50th and 95th quantile) for responses of pair spawning frequencies per territory and day (a) and female sizes of pair spawners per territory and day (b) to correspondent group spawning quantities per territory and day. Only the 95th quantile is significant for both graphs (p < 0.01)
A linear regression suggested that group spawning activities were significantly positively correlated with increasing densities of sixbar individuals (individuals per 50 m2) at the spawning site (linear regression, r2 = 0.0825, F1,111 = 9.985, p = 0.002), whereas pair spawning activities did not show any significant associations with spawning site density (Fig. 5).
Linear regression for responses of pair spawning (white circles) and group spawning (black circles) activities (number of spawning events per territory and day) to quantities of females and males (number of individuals per 50 m2) at the spawning site. Only group spawning events were significantly correlated to densities at the spawning site (p = 0.002)
Variation in male streaking strategies
Streaking attempts were predominantly influenced by moon phase (Table 5), and were more common around the last quarter moon and new moon period (GLMER, cosθ: estimate ± SE = 0.57 ± 0.14, p < 0. 001, marg. r2 = 44.2 Fig. 6a). Frequencies of streaking attempts (evidenced by the numbers of chases initiated by the focal TP) dropped markedly around first quarter moon and were minimal at the full moon. Streaking attempts were also less common at more sheltered territories (GLMER, estimate ± SE = − 0.37 ± 0.06, p < 0. 001, marg. r2 = 6.8, Table 5). Because successful streaking or sneaking events were extremely rare, (e.g., only 3.5 and 0.7 percent, respectively, out of 649 bouts of spawning), statistical evaluations variation in these strategies were not possible.
The sizes of unsuccessful streaking males (i.e., IP males that attempted a sneak spawn but were chased away by the TP) were also strongly related to the lunar cycle. Average size of unsuccessful streakers significantly increased through the full moon and reached a maximum size at the third quarter moon (GLM, cosθ: estimate ± SE = − 0.61 ± 0.16, p < 0. 001, marg. r2 = 50.3, Fig. 6b). Sizes of unsuccessful streakers decreased around new moon and through the first quarter moon. Site and proximity to the reef edge did not account for any significant variation in the sizes of streakers males (Table 5).
Discussion
Sixbar wrasse exhibit substantial variability of reproductive investment decisions that appear to depend strongly upon the lunar cycle. Investment decisions also vary with location, body size, and reproductive status of individuals. Frequencies of pair spawning, group spawning, and streaking attempts all significantly increased around the new moon. Frequencies of these activities were also greatest in the most exposed territories (i.e., those located closest to the reef edge). Smaller individuals of both sexes curtailed reproduction during the full moon and third quarter moon, while larger individuals reproduced more consistently across the lunar month. Size-dependent reproductive decisions appear to mediate frequencies of specific reproductive tactics such as pair spawning, group spawning and streaking strategies.
Temporal variation in female reproductive strategies
Temporal patterns of pair spawning indicate that larger females spawn throughout much of the lunar month (with elevated activities around new moon), whereas smaller individuals tend to concentrate their reproductive investment around the new moon period. Intraspecific variation in spawning activities may reflect size-dependent trade-offs between reproduction and growth (Henson and Warner 1997; Warner 1998; Heino and Kaitala 1999; Alonzo and Warner 2000; Clark and Mangel 2000). Typically, reproductive activities can entail substantial expenses in energy (Nielsen et al. 2012; McBride et al. 2015), as adults must divert their available resources to gametogenesis, migration, and reproductive behaviour (Roff 1991; Shapiro et al. 1994; Warner 1995; McBride et al. 2015). Consequently, energy investment into reproduction can come at the cost of reduced somatic growth and associated survival potential.
As fecundity and survival potential significantly increases with size in iteroparous protogynous species (Warner 1984a; Schultz and Warner 1991; Munday et al. 2006), smaller females may prioritize growth over reproduction (van Rooij et al. 1995; Warner 1998) to increase their probability of surviving to spawn at a larger size. Environmental conditions (i.e., hydrodynamics, putative benefits to offspring growth and survival) associated with the new moon may be sufficiently beneficial to warrant spawning even at a small size. Larger females may have more excess energy available for investment into gametogenesis and migratory behaviour, and thus may be able to afford more frequent reproduction (Warner 1998). Additionally, larger females may be more willing to engage in reproductive activities, because expected fitness rewards may exceed relative risks associated with reproduction (mortality risk decreases with size; Warner 1984b, 1998; Hixon and Beets 1993; DeMartini et al. 2005; Welsh et al. 2013).
Increased reproductive activities during new moon suggest that this time may provide a selective advantage for reproduction (e.g., synchronized reproduction, higher adult or offspring survival; Johannes 1978; Robertson et al. 1990; Claydon et al. 2014; Shima et al. 2018, 2021). So why does selection favour continuous reproduction outside of favourable environmental conditions for larger individuals? Larval survival in oceanic environments varies substantially due to a variety of unpredictable environmental selection pressures (Warner 1997a; Searcy and Sponaugle 2000; Shima et al. 2018). Consequently, larger females with sufficient energetic resources may increase their fitness, by spreading their reproductive effort over an extended period, to bet-hedge against unpredictable variation in larval survivorship (Wilbur and Rudolf 2006; Shima et al. 2021). This strategy may maximize fitness because the chances that at least some offspring surviving to recruitment may be increased; particularly if pelagic larvae with an inauspicious birthdate can actively alter their developmental trajectories (as indicated by Shima et al. 2020).
Temporal variation in male reproductive strategies
Initial phase males
Initial phase males (IP males) increased their reproductive effort during the new moon but showed limited reproductive activities during the rest of the lunar month. These findings align with our hypothesis that reproductive investment patterns of small initial phase males should mirror the investment strategies of smaller females (see also Warner 1975, 1998). Because small males are essentially always operating in conditions of sperm competition, they must invest a large proportion of their excess energy into sperm production (testes weights of IP males are much bigger than those of TP males; Shapiro et al. 1994; Taborsky 1998; Molloy et al. 2007). Thus, current reproduction is limited by energy, and this implies that IP males might not be able to gather and reallocate enough energetic resources to reproduce over the entire month (Hoffman 1983; Warner 1984b). Additionally, initial phase males face many future reproductive events and have the potential to become territorial males with exclusive access to females. Hence, competitively inferior males should direct a significant portion of their time and energy to foraging activities, at the cost of continuous reproduction, to facilitate growth and associated survival to a competitive size (Hoffman 1983; Warner 1984b, 1998).
Given that 95% of all streaking attempts were unsuccessful, streaking alone appears to be an extremely ineffective reproductive strategy (although qualitative evaluation of the data suggest that streaker males may have been slightly more successful around new moon). So why do initial phase males (i.e., presumably, an alternative reproductive strategy) exist, if at first glance, their probability of successful fertilization of eggs appears to be vanishingly small relative to females? The answer might lie in their behavioural flexibility to switch between alternative tactics during new moon. Efficacy of territory defence by a TP dramatically declined with increasing density of IP males, and this was most commonly observed around the new moon. At a certain density threshold, initial phase males appeared to overwhelm territory owners, and most matings went to IP males in group spawning assemblages. By periodically employing group spawning strategies, IP males might, therefore, attain a similarly high reproductive success relative to females (Warner 1975; Warner and Hoffman 1980a, b; Henson and Warner 1997; Warner 1998).
Group spawning as an alternative tactic may be evolutionarily maintained in a population if females are either not discriminating between male reproductive phenotypes or are actively choosing between male alternatives if direct fitness benefits occur (Warner and Hoffman 1980a, b; Alonzo and Warner 2000; Taborsky and Brockmann 2010). Quantile regression analyses indicated that differently sized females may vary in their choice of male alternatives: smaller females appeared to prefer multi-male group spawning assemblages, while larger females showed a tendency towards pair spawning activities. These findings coincide with behavioural observations on the closely related bluehead wrasse Thalassoma bifasciatum. Female blueheads tend to consistently reproduce at group spawning sites when small but shift to pair spawning activities when reaching larger sizes (Warner and Robertson 1978). It is still not exactly known why females choose one male phenotype over the other dependent on their physiological condition. Potentially, spawning in large groups may reduce mortality risk for smaller females via dilution effects and earlier predator detection (Robertson 1983), while larger females with lower size-related mortality risk may accrue greater fitness by reproducing with a high-quality male (Henson and Warner 1997; Alonzo and Warner 2000). Irrespective of the proximate drivers, size-dependent variation in female choice might maintain a stable co-existence of the two male reproductive phenotypes (initial phase males and terminal phase males) in a sex-changing population (Henson and Warner 1997; Alonzo and Warner 2000; Taborsky and Brockmann 2010).
Territorial males
Because of their size-related ability to continuously occupy and control a mating site over an extended time, territorial males accrue a substantially higher lifetime reproductive success relative to females and IP males (Warner 1984a; Warner et al. 1995). However, their fitness only maximizes if they successfully exclude initial phase males from the mating site. Allocation patterns of terminal phase males thus fundamentally differ from females and initial phase males (Hoffman 1983; Warner and Hoffman 1980a; Taborsky 1998). As their mating success depends on mating site control, territorial males expend most of their energy on aggressive behaviour (their rate of aggression is much higher than in any other reproductive phenotype, see also Warner and Hoffman 1980a). Consequently, TP males show the lowest growth rates and have relatively small testes in comparison to gonads of females and initial phase males (Hoffman 1983; Warner and Hoffman 1980a, b; Taborsky 1998). During the new moon, mating success of territorial males sometimes dramatically declined with increasing numbers of initial phase males at the spawning site. At higher densities, territorial males shifted most of their time towards territory defence, which detracted from time available for mating activities. Even after group spawning assemblages predominated the spawning site, territorial males avoided any participation in group spawning events, and continued to vigorously defend their mating territory. Group spawning, with chronic sperm competition, would yield minimal reproductive success for terminal phase males due to their relatively small testis size (Warner et al. 1995). Thus, territorial males are predicted to prioritize territory defence, because loss of a territory would equal almost complete loss of fertilization success (Hoffman 1983; Warner et al. 1995). Despite reduced mating success during the new moon, continuous access to female mates outside the new moon period probably overcompensates for any short-term losses in reproduction.
Spatial variation in female reproductive strategies
Highly iteroparous females tend to spawn in locations that maximize immediate survivorship of offspring (Johannes 1978; Colin 1992; Whaylen et al. 2004; Claydon et al. 2014). Hence, site quality rather than male quality is the object of female choice (Warner 1998). Preferred mating territories are mostly located in relatively exposed areas that promote dispersal of fertilized eggs away from benthic predators (Johannes 1978; Lobel 1978; Sancho et al. 2000; Claydon et al. 2014). The findings of this study align with the general trend (highest pair spawning activity in exposed sites), however, mating site preferences exhibited great among-individual variation: smaller females preferred to spawn in sheltered territories, while larger females showed a higher affinity for exposed territories. As females have to ascend to the surface to release their gametes, any spawning event critically exposes them to pelagic predators. Adults that spawn in territories close to the reef shelf are at particularly high risk to attacks from piscivores that hunt along the reef edge (Johannes 1978; Robertson and Hoffman 1977; Robertson 1983; Moyer 1987; Sancho et al. 2000). In a few instances, we observed predation attempts (mostly from trevallies) on pair spawning females within exposed territories. Highly iteroparous females in a sex-changing population are predicted to prioritize growth and their own survival over any potential short-term gain in fitness (Warner 1998). Intraspecific variation in spawning site choice may thus be mediated by size-dependent trade-offs between offspring and maternal survival potential (Shibuno et al. 1993; Warner 1998). Since predation risk varies inversely with size, smaller females may choose territories in more sheltered areas of the reef to increase their own probability of survival at the cost of increased offspring survival. In contrast, larger females with reduced predation risk may be more willing to reproduce in exposed territories that facilitate offspring dispersal.
Conclusion
Collectively, these results highlight the diversity of life history strategies present within a species of coral reef fish, and suggest that optimal tactics change with ontogeny, and across the lunar month. Smaller males and females may substantially increase their fitness if they direct most of their energy towards growth and target their reproductive effort using external clues that may indicate the most profitable times (e.g., around the new moon period) and places (e.g., proximity to the reef edge). The relative benefits of this targeted strategy may change as individuals grow larger, because they have more energy available for reproductive investment and a higher, size-related survival potential. Our study also shows that intraspecific investment decisions in relation to an environmental context determine the prevailing reproductive mode at a spawning site; if smaller males and females reduce their reproductive effort, pair spawning and streaking strategies predominate. If they shift resource allocations towards reproductive activities in response to environmental cues, higher abundances at the spawning site lead to group spawning activities. Variation in female choice might thereby act as a mechanism for maintaining these two reproductive modes (pair spawning and group spawning) in a population.
Data availability
Data and/or code can be made available upon request.
References
Aldenhoven JM (1986) Different reproductive strategies in a sex changing coral reef fish Centropygebicolor (Pomacanthidae). Aust J Mar Freshw Res 37:353–360. https://doi.org/10.1071/MF9860353
Alonzo SH, Warner RR (2000) Female choice, conflict between the sexes and the evolution of male alternative reproductive behaviours. Evol Ecol Res 2(2):149–170
Bates D, Maechler M, Bolker B, Walker S (2013) lme4: Linear mixed-effects models using Eigen and S4 classes. R package version 10–4. https://doi.org/10.1111/mec.12785/abstract.
Clark CW, Mangel M (2000) Dynamic state variable models in ecology: methods and applications. Oxford University Press, New York
Claydon J (2004) Spawning aggregations of coral reef fishes: characteristics, hypotheses, threats and management. Oceanogr Mar Biol 42:265–302. https://doi.org/10.1201/9780203507810.ch7
Claydon J, McCormick MI, Jones GP (2014) Multispecies spawning sites for fishes on a low-latitude coral reef: spatial and temporal patterns. J Fish Biol 84:1136–1163. https://doi.org/10.1111/jfb.12355
Colin PL, Clavijo IE (1988) Spawning activity of fishes producing pelagic eggs on a shelf edge coral reef, southwestern Puerto Rico. Bull Mar Sci 43:149–179
deBruyn AMH, Meeuwig JJ (2001) Detecting lunar cycles in marine ecology: periodic regression versus categorical ANOVA. Mar Ecol Prog Ser 214:307–310. https://doi.org/10.3354/meps214307
DeMartini EE, Friedlander AM, Holzwarth SR (2005) Size at sex change in protogynous labroids, prey body size distributions, and apex predator densities at NW Hawaiian atolls. Mar Ecol Prog Ser 297:259–271. https://doi.org/10.3354/meps297259
Domeier ML, Colin PL (1997) Tropical reef fish spawning aggregations defined and reviewed. Bull Mar Sci 60:698–726
Grömping U (2006) Relative importance for linear regression in R: the package relaimpo. J Stat Softw 17:1–27. https://doi.org/10.18637/jss.v017.i01
Heino K, Kaitala V (1999) Evolution of resource allocation between growth and reproduction in animals with indeterminate growth. J Evol Biol 12(3):423–429. https://doi.org/10.1046/j.1420-9101.1999.00044.x
Henson SA, Warner RR (1997) Male and female alternative reproductive behaviors in fishes: a new approach using intersexual dynamics. Annu Rev Ecol Syst 28:571–592. https://doi.org/10.1146/annurev.ecolsys.28.1.571
Hixon MA, Beets JP (1993) Predation, prey refuges, and the structure of coral-reef fish assemblages. Ecol Monogr 63:77–101. https://doi.org/10.2307/2937124
Hoffman SG (1983) Sex-related foraging behaviour in sequentially hermaphroditic hogfishes (Bodianus spp.). Ecology 64:798–808. https://doi.org/10.2307/1937203
Johannes RE (1978) Reproductive strategies of coastal marine fishes in the tropics. Environ Biol Fishes 3:65–84. https://doi.org/10.1007/BF00006309
Koenker R (2011) quantreg: Quantile Regression. R Package Version 4.62. http://CRAN.Rproject.org/package=quantreg
Lindeman RH, Merenda P, Gold R (1980) Introduction to bivariate and multivariate analysis. Scott, Foresman, Glenview, IL
Lobel PS (1978) Diel, lunar, and seasonal periodicity in the reproductive behavior of the pomacanthid fish, Centropygepotteri, and some other reef fishes in Hawaii. Pac Sci 32:193–207
Lenth RV (2018) Emmeans: estimates marginal means, aka least-squares means. R package version 1.1.3. https://CRAN.R-project.org/package=emmeans
Mcbride RS, Somarakis S, Fitzhugh GR, Albert A, Yaragina NA, Wuenschel MJ, Alonso-Fernández A, Basilone G (2015) Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish Fish 16(1):23–57. https://doi.org/10.1111/faf.12043
Mitterwallner P (2020) Reproductive timing and investment decisions of a protogynous, hermaphroditic coral reef fish species. Dissertation, Victoria University of Wellington
Mitterwallner P, Shima JS (2022) The relative influence of environmental cues on reproductive allocation of a highly iteroparous coral reef fish. Coral Reefs. https://doi.org/10.1007/s00338-022-02239-6
Molloy PP, Goodwin NB, Côté IM, Reynolds JD, Gage MJG (2007) Sperm competition and sex change: a comparative analysis across fishes. Evolution 61(3):640–652. https://doi.org/10.1111/j.1558-5646.2007.00050.x
Moyer JT (1987) Quantitative observations of predation during spawning rushes of the labrid fish Thalassoma cupido at Miyake-jima, Japan. Jpn J Ichthyol 34:76–81. https://doi.org/10.11369/jji1950.34.76
Munday PL, Buston PM, Warner RR (2006) Diversity and flexibility of sex-change strategies in animals. Trends Ecol Evol 21(2):89–95. https://doi.org/10.1016/j.tree.2005.10.020
Muñoz RC, Warner RR (2003) A new version of the size-advantage hypothesis for sex change: incorporating sperm competition and size-fecundity skew. Am Nat 161(5):749–761. https://doi.org/10.1086/374345
Nemeth RS, Blondeau J, Herzlieb S, Kadison E (2007) Spatial and temporal patterns of movement and migration at spawning aggregations of red hind, Epinephelusguttatus, in the U.S. Virgin Islands Environ Biol Fishes 78:365–381. https://doi.org/10.1007/s10641-006-9161-x
Nielsen JR, Lambert G, Bastardie F, Sparholt H, Vinther M (2012) Do Norway pout (Trisopterusmarkii) die from spawning stress? Mortality of Norway pout in relation to growth, sexual maturity, and density in the North Sea, Skagerrak, and Kattegat. ICES J Mar Sci 69:197–207. https://doi.org/10.1093/icesjms/fss001
Petersen CW, Warner RR, Cohen S, Hess HC, Sewell AT (1992) Variable pelagic fertilization success: implications for mate choice and spatial patterns of mating. Ecology 73:391–401. https://doi.org/10.2307/1940747
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rideout RM, Rose GA, Burton MP (2005) Skipped spawning in female iteroparous fishes. Fish Fish 6:50–72. https://doi.org/10.1111/j.1467-2679.2005.00174.x
Robertson DR (1983) On the spawning behavior and spawning cycles of eight surgeon fishes (Acanthuridae) from the Indo-Pacific. Environ Biol Fishes 9:193–223
Robertson DR, Hoffman SG (1977) The roles of female mate choice and predation in the mating systems of some tropical labroid fishes. Zeitschrift für Tierpsychologie 45:298–320. https://doi.org/10.1111/j.1439-0310.1977.tb02123.x
Robertson DR, Petersen CW, Brawn JD (1990) Lunar reproductive cycles of benthic brooding reef fishes: reflections of larval biology or adult biology? Ecol Monogr 60:311–329. https://doi.org/10.2307/1943060
Roff DA (1991) Life history consequences of bioenergetic and biomechanical constraints on migration. Integr Comp Biol 31(1):205–216. https://doi.org/10.1093/icb/31.1.205
Sancho G, Solow AR, Lobel PS (2000) Environmental influences on the diel timing of spawning in coral reef fishes. Mar Ecol Prog Ser 206:193–212. https://doi.org/10.3354/meps206193
Schultz ET, Warner RR (1991) Phenotypic plasticity in life-history traits of female Thalassoma bifasciatum (Pisces: Labridae): 2. Correlation of fecundity and growth rate in comparative studies. Environ Biol Fishes 30(3):333–344. https://doi.org/10.1016/B978-0-12-817996-3.00012-8
Searcy SP, Sponaugle S (2000) Variable larval growth in a coral reef fish. Mar Ecol Prog Ser 206:213–226. https://doi.org/10.3354/meps206213
Shapiro DY (1991) Intraspecific variability in social systems of coral reef fishes. In: Sale P (ed) The ecology of fishes on coral reefs. Academic Press, San Diego, pp 331–355
Shapiro DY, Marconato A, Yoshikawa T (1994) Sperm economy in a coral reef fish, Thalassomabifasciatum. Ecology 75:1334–1344. https://doi.org/10.2307/1937458
Shibuno T, Gushima K, Kakuda S (1993) Female spawning migrations of the protogynous wrasse, Halichoeresmarginatus. Jpn J Ichthyol 39:357–362
Shima JS, Noonburg EG, Swearer SE, Alonzo SH, Osenberg CW (2018) Born at the right time? A conceptual framework linking reproduction, development, and settlement in reef fish. Ecology 99:116–126. https://doi.org/10.1002/ecy.2048
Shima JS, Swearer SE (2019) Moonlight enhances growth in larval fish. Ecology. https://doi.org/10.1002/ecy.2563
Shima JS, Osenberg CW, Alonzo SH, Noonburg EG, Mitterwallner P, Swearer SE (2020) Reproductive phenology across the lunar cycle: parental decisions, offspring responses, and consequences for reef fish. Ecology 101:e03086. https://doi.org/10.1002/ecy.3086
Shima JS, Osenberg CW, Noonburg EG, Alonzo SH, Swearer SE (2021) Lunar rhythms in growth of larval fish. Proc R Soc B 288:20202609. https://doi.org/10.1098/rspb.2020.2609
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Taborsky M, Brockmann HJ (2010) Alternative reproductive tactics and life history phenotypes. In: Kappeler P (ed) Animal behaviour: evolution and mechanisms. Springer, pp 537–586
Taborsky M, Hudde B, Wirtz P (1987) Reproductive behaviour and ecology of Symphodus (Crenilabrus) ocellatus, a European wrasse with four types of male behaviour. Behaviour 102:82–118
Taborsky M (1998) Sperm competition in fish: ‘Bourgeois’ males and parasitic spawning. Trends Ecol Evol 13:222–227. https://doi.org/10.1016/S0169-5347(97)01318-9
Van Rooij JM, Bruggermann JH, Videler JJ, Breeman AM (1995) Plastic growth of the herbivorous reef fish Sparisomaviride: field evidence for a trade-off between growth and reproduction. Mar Ecol Prog Ser 122:93–106. https://doi.org/10.3354/meps122093
Warner RR (1975) The adaptive significance of sequential hermaphroditism in animals. Am Nat 109(965):61–82. https://doi.org/10.1086/282974
Warner RR (1984a) Mating behavior and hermaphroditism in coral reef fishes: the diverse forms of sexuality. Am Sci 72:128–136
Warner RR (1984b) Deferred reproduction as a response to sexual selection in a coral reef fish: a test of the life historical consequences. Evolution 38(1):148. https://doi.org/10.2307/2408554
Warner RR (1995) Large mating aggregations and daily long-distance spawning migrations in the bluehead wrasse, Thalassomabifasciatum. Environ Biol Fishes 44:337–345. https://doi.org/10.1007/BF00008248
Warner RR (1997a) Evolutionary ecology: how to reconcile pelagic dispersal with local adaptation. Coral Reefs 16:115–120. https://doi.org/10.1007/s003380050247
Warner RR (1997b) Sperm allocation in coral reef fishes—strategies for coping with demands on sperm production. Bioscience 47(9):561–564. https://doi.org/10.2307/1313162
Warner RR (1998) The role of extreme iteroparity and risk avoidance in the evolution of mating systems. J Fish Biol 53:82–93. https://doi.org/10.1111/j.1095-8649.1998.tb01019.x
Warner RR, Hoffman SG (1980a) Population density and the economics of territoriality in a coral reef fish. Ecology 61:772–780. https://doi.org/10.2307/1936747
Warner RR, Robertson DR (1978) Sexual patterns in the labroid fishes of the western Caribbean, I the Wrasses (Labroidae). Smithsonian Institution Press, Washington
Warner RR, Hoffman SG (1980b) Local population size as a determinant of mating system and sexual composition in two tropical marine fishes (Thalassoma spp.). Evolution 34:508–518. https://doi.org/10.2307/2408220
Warner RR, Shapiro DY, Marcanato A, Petersen CW (1995) Sexual conflict: males with highest mating success convey the lowest fertilization benefits to females. Proc R Soc B Biol Sci 262(1364):135–139. https://doi.org/10.1098/rspb.1995.0187
Welsh JQ, Goatley CHR, Bellwood DR (2013) The ontogeny of home ranges: evidence from coral reef fishes. Proc R Soc B Biol Sci 280(1773):1–7
Wilbur HM, Rudolf VHW (2006) Life-history evolution in uncertain environments: bet hedging in time. Am Nat 168:398–411. https://doi.org/10.1098/rspb.2013.2066
Whaylen L, Pattengill-Semmens CV, Semmens BX, Bush PG, Boardman MR (2004) Observations of a Nassau grouper, Epinephelus striatus, spawning aggregation site in Little Cayman, Cayman Islands, including multi-species spawning information. Environ Biol Fishes 70:305–313. https://doi.org/10.1023/B:EBFI.0000033341.57920.a8
Acknowledgements
P. Caie, K. Hillyer assisted with data collection. Research grants from Marsden Fund (VUW1503, 2016–2020), and Victoria University of Wellington provided funding. Victoria University Coastal Ecology Lab and the UC Gump Research Station provided essential logistic support. S. Swearer provided constructive advice on initial sampling design.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was supported by the Marsden Fund (VUW1503, 2016-2020), and the Victoria University of Wellington.
Author information
Authors and Affiliations
Contributions
This study was designed by PM and JS. Data collection was carried out by PM. Data analysis was performed by PM. PM drafted the manuscript, with all authors making significant contributions to writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This research was supported by the Marsden Fund (VUW1503, 2016-2020), and the Victoria University of Wellington. The authors declare that all applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study have been followed and all necessary approvals have been obtained. All research was conducted under protocols approved by Victoria University of Wellington’s Animal Ethics Committee (permit numbers: 22038, and 26378). Field work in French Polynesia was conducted under research permits issued by the Delegation a la Recerche (de la Polynesie Francaise).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: K. D. Clements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mitterwallner, P., Shima, J.S. Influence of the lunar cycle and spatial gradients on size-dependent male and female reproductive investment decisions of a protogynous reef fish. Mar Biol 169, 129 (2022). https://doi.org/10.1007/s00227-022-04109-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04109-9