Abstract
To understand the demographic responses of green turtles to seagrass decline, we examined a data set from study of a mixed-stock foraging aggregation of immature green turtles, Chelonia mydas, collected in Bermuda (32o18’N, − 64o46’W) over five decades. Average turtle size (SCLmin) and mass declined by 22.3% and 58.2%, respectively. Aggregation size structure shifted to smaller sizes and now consists of more small turtles and fewer large turtles. Density (turtles ha−1) increased significantly but biomass (kg ha−1) remained unchanged and low compared to C. mydas biomass observed elsewhere. Green turtles exhibited reduced site fidelity during two portions of the study period, suggesting increased foraging effort. Reduction in turtle body condition index and seagrass coverage occurred from offshore to inshore. Changes in aggregation composition and behavior were consistent with expectations given a documented decline in seagrass availability, combined with increased output from source rookeries. Apparent response to resource decline is traced back to 1976, well before seagrass loss was first documented. Green turtles and their primary food source (Thalassia testudinum) are at the northern limit of their range in Bermuda, where seagrasses would be expected to have a reduced tolerance for natural grazing pressure and increased susceptibility to synergistic stressors, especially temperature, bioturbation and phosphorus limitation. Our results suggest that synergistic stressors, and not green turtles alone, have produced the observed reduction in seagrasses on the Bermuda Platform. Given that seagrass declines have been reported worldwide, our findings may suggest how green turtles will respond elsewhere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chelonia mydas and seagrasses
The relationship between the green turtle (Chelonia mydas) and seagrasses has been a subject of concentrated study by marine biologists for decades. The reliance of this marine megaherbivore on seagrasses, at least in some portions of its distribution, has received considerable attention from ecological (Mortimer 1981; Bjorndal 1982, 1985; Moran and Bjorndal 2005; Gulick et al. 2020, 2021a; Johnson et al. 2019; Esteban et al. 2020), behavioral (Bjorndal 1980; Ogden et al. 1983; Gulick et al. 2021b), and physiological perspectives (Bjorndal 1979, 1982, 1997). In recent years, an increasing body of literature indicates that seagrasses are declining worldwide (Orth et al. 2006; Waycott et al. 2009; Grech et al. 2012) due to a complex set of mostly anthropogenic factors (Nelson 2009). In addition to concern about seagrass ecosystem decline and loss of ecosystem services (Lal et al. 2010; Scott et al. 2018), there is concern about how the loss of seagrasses might impact the ongoing recovery of green turtles that rely on seagrasses as a primary food source (Fourqurean et al. 2010; Christianen et al. 2014; Burgett et al. 2018; Gulick et al. 2020). Although there is ample data to suggest that green turtles can use alternative resources to seagrasses (Brand-Gardner et al. 1999; Lemons et al. 2011; Burgett et al. 2018; Esteban et al. 2020), significant decline in seagrass can be expected to produce changes in the demographics and ecology of herbivores that rely on them. The focus of this paper is a long-term study by the Bermuda Turtle Project (BTP) that documents significant demographic changes in a mixed-stock, developmental (non-adult) foraging aggregation of C. mydas at a site where seagrass declines (primarily Thalassia testudinum) have been documented (Murdoch et al. 2007; Fourqurean et al. 2010, 2019; Manuel et al. 2013; this study). This study is timely because seagrasses worldwide have shown significant declines just as green turtle rookeries have started to recover after being decimated by the beginning of the twentieth century (McClenachan et al. 2006). The foraging aggregation we studied is within the North Atlantic Distinct Population Segment (DPS) and receives some input from the South Atlantic DPS (BTP unpubl data). Both DPSs include multiple rookeries that are showing signs of recovery (Seminoff et al. 2015).
Green turtle life cycle
The green turtle has a complex life cycle that includes the use of different food resources and foraging strategies at different life stages. After hatching, green turtles enter an epipelagic or oceanic stage, during which there may be continuous travel and an omnivorous diet in pelagic drift habitats (e.g., rafts of Sargassum spp. in the Atlantic) at or near the ocean surface (Witherington et al. 2012; Hardy et al. 2018; Mansfield et al. 2021). At about 25 cm (SCLmin), most individuals in the West Atlantic settle on benthic foraging grounds (Meylan et al. 2011) at which seagrasses or marine algae are their primary food source (Bjorndal 1997). The individuals that occupy foraging areas dominated by post-pelagic immatures typically belong to multiple reproductive populations (Lahanas et al. 1998; Bass and Witzell 2000; Luke et al. 2004; Meylan et al. 2011) and are best considered mixed-stock “aggregations” rather than “populations” (Bjorndal et al. 2005). The sites that these immature aggregations occupy, “benthic developmental foraging grounds,” act as long-term residency sites for immatures during an extended period of their development. Turtles making up these aggregations typically depart from developmental sites before they mature (Meylan et al. 2011; Bjorndal et al. 2019). Other, geographically separate seagrass or marine algae-dominated foraging grounds, usually closer to natal beaches, serve as additional developmental sites or foraging areas for large immatures and adults from which adults regularly undertake reproductive migrations. Variation in this general pattern is discussed by Musick and Limpus (1997) and Meylan et al. (2011).
This study covers a period during which multiple green turtle rookeries in the West Atlantic increased in size (Chaloupka et al. 2008; Seminoff et al. 2015; Mazaris et al. 2017). Long-term protection of C. mydas in Bermuda and elsewhere within the Atlantic likely contributed to this increase. Green turtles in Bermuda received complete protection starting in 1972 (Sarkis and Outerbridge 2014), and turtles tagged in Bermuda are known to have joined nesting populations in Costa Rica, Mexico and Florida (Meylan et al. 2014, 2020). Genetic data suggest that turtles that make up this aggregation may belong to nesting populations from as far away as Guinea Bissau (BTP unpubl data). Thus, the health and survivorship of immature green turtles that complete a large part of their life cycle in Bermuda play a role in the demographics of multiple reproductive populations in at least 2 DPSs (Seminoff et al. 2015).
Bermuda as a C. mydas study site
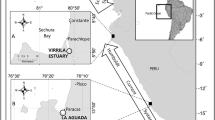
Bermuda is an isolated seamount located ~ 1050 km east of Cape Hatteras, North Carolina, east of the Gulf Stream, within the central north Atlantic (32o18’N, -64o46’W). A 50-year-long study of C. mydas by the BTP provides the opportunity to describe changes in a developmental aggregation over a period during which there has been documented decline in its seagrass resource base. Bermuda is an excellent site at which to observe the impact of seagrass decline on this immature life stage for multiple reasons. There are no adult green turtles present at any time which eliminates any possible influence of adult pressures on local seagrass resources. In addition, the Bermuda Platform is isolated from other foraging grounds by more than 1000 km so there are no nearby resources to be taken into consideration. Thirdly, there has been no green turtle fishery in Bermuda since 1972 (Sarkis and Outerbridge 2014) so harvest need not be considered for nearly all of the study period. Finally, the condition of seagrass meadows (dominated by T. testudinum but including Syringodium filiforme, Halodule sp. and Halophila decipiens) is well studied (Murdoch et al. 2007; Manuel et al. 2013). As of 1997, seagrass coverage in Bermuda was estimated to be ~ 2100 ha (Vierros et al. 2002) but has declined markedly since that date (Murdoch et al. 2007; Manuel et al. 2013; this study).
The extensive data set on green turtles collected by the BTP since 1968 allows examination of changes in the size structure, density (turtles ha−1), biomass (kg ha−1), body condition, and site fidelity over decades. Qualitative assessments of seagrass meadow condition made during about half of the sampling events (383 of 743 sets of an entrapment net) between 1993 and 2018 provide an index of seagrass condition at the sites where turtles were sampled. These data complement previous studies of seagrass abundance and distribution on the Bermuda Platform (Vierros et al. 2002; Murdoch et al. 2007; Manuel et al. 2013).
Decline of seagrasses and green turtle response
Decline in seagrasses appears to be an extremely complex process as it can result from a suite of at least 11 different stressors, including six abiotic and five biotic limiting factors (Nelson 2009) all of which can be influenced by anthropogenic interactions. Recent work in Australia suggests that “synergistic stressors”, including increases in water temperature and flood-induced turbidity, have negatively impacted seagrasses (Fraser et al. 2014; Kendrick et al. 2019). Climate change is thought to lower resilience of seagrasses, as temperature effects appear to magnify the impacts of synergistic stressors, especially at the edge of their geographic range (Fraser et al. 2014; Arias-Ortiz et al. 2018). Seagrasses in Bermuda are at the northern limit of their range and are restricted to shallow water (Manuel et al. 2013), making them more susceptible to temperature extremes. Clearly, megaherbivores play a key role in structuring seagrass meadows (e.g., Scott et al. 2018; Gulick et al. 2020; Christianen et al. 2021). Green turtles and seagrasses have coexisted for millions of years (Dodd et al. 1992) and perhaps 10s of millions of years (Ivany et al. 1990). Thus, there should exist natural mechanisms by which these grazers respond to the availability of resources, and seagrasses respond to fluctuations in grazing pressure. Our long-term work in Bermuda provides an opportunity to investigate changes in an important green turtle foraging aggregation and examine the expectation that such aggregations should show predictable responses to decline in seagrass forage availability.
Materials and methods
Study area and sampling
The BTP has sampled green turtles on seagrass meadows across the Bermuda Platform from 1968 to the present. All sampling was done using an entrapment net method (Meylan et al. 2011) in which a 6.1 m deep net of 10 cm bar mesh with continuous lead line and float line was set on seagrass meadows in a circle or from shore to shore. Net length varied from 614 to 396 m. Snorkelers swam the net removing captured turtles and placing them in a catch boat. Turtles were then transferred to a larger research vessel for data and sample collection. Turtle size and mass data were collected from all captures, however, from 1968 to 1991, specific set data were not associated with turtle capture data, limiting the utility of information from these early years. From 1992 to 2018, the BTP deployed an entrapment net 743 times at 42 locations. During this period, basic habitat data, including GPS coordinates of set locations, depth at the approximate center of the set, and water temperature were available for nearly all sets. Association of capture data with set data allowed calculation of catch per unit effort (CPUE) and density as well as biomass per unit effort (BPUE) and biomass. For 51.5% of these sets, snorkelers made a qualitative assessment of the benthic habitat, especially the seagrasses, as they swam the circumference of the net to remove turtles; these observations were recorded in the project logbook. We later converted these assessments to a qualitative seagrass index by scoring them as 0–5 based on the specific terminology used in the recorded descriptions and employing a scale approximating that of Murdoch et al. (2007) (Table S-1). Quantification of seagrass coverage for our index categories was available for 34 sets of the net made during 1993 and 1994 (Vierros 1999; Vierros et al. 2002). Quantification resulted from analysis of 10–15, 0.5-m2 quadrats for each of 34 sets that included estimates of seagrass coverage (sum of % vegetative cover contributed by T. testudinum and S. filiforme) (Table S-1). Turtle size used throughout this paper is reported as straight carapace length measured in cm from nuchal notch to pygal notch (SCLmin of Bolten, 1999). Turtles were weighed to the nearest 0.1 kg using mechanical scales until 1992, and an electronic scale after that date.
Physical characteristics and sampling records for 20 sites (named seagrass meadows) sampled 10 times or more between 1992 and 2018 are given in Table 1. Those sites represent a cross section of foraging habitats for green turtles in Bermuda and were the location of 90.7% of the sets of the entrapment net made during all months between 1992 and 2018. They are distributed across the Platform (Fig. 1) from the shoreline of the main islands to 11.2 km from the nearest shoreline and vary from 2.0 to 5.8 m in average depth. The sites varied in their degree of isolation, from those that are distant from all other seagrass meadows (North Rock) to sites that are essentially continuous (Wreck Hill and Tudor Hill, Grotto Bay and Ferry Reach). The number of times that each site was sampled per year was limited to avoid affecting turtle behavior. Data from a subset of 15 sites sampled at least 10 times between 22 July and 25 August from 1992 to 2018 provided a sample of “August” sets that were used in analyses for which we sought to minimize any potential seasonal effects (Table 2).
Analytical methods
Preliminary analyses suggested that sampling sites at roughly the same distance offshore from the main islands showed similar patterns of turtle use and seagrass decline. Thus, we categorized sampling sites as inshore (along or within the main islands of Bermuda), nearshore (immediately across one deep channel from the main islands), or offshore (at least 1.5 km from the main islands). These sampling site categories are given in Table 1 and used in analyses of site-specific changes. Changes in the absolute size of a green turtle developmental aggregation can result from variation in immigration, emigration and survivorship (Bjorndal et al. 2005). Given the complexity of distinguishing local movement out of our specific sampling sites on the Platform from emigration or mortality, we have chosen to use absolute measures of density and biomass on individual seagrass meadow sampling sites as indicators of change in the “size” of the aggregation. For sets of the entrapment net made from 1992 to 2018, we calculated the area enclosed by the entrapment net to determine CPUE and BPUE for a standardized area (3.0 ha). Given the known area, we could then compute density (turtles ha−1) and biomass (kg ha−1). The area of the set was determined by knowing the length of the net and considering it to be the circumference of a circle. When the length of the net changed, and for sets made from point-to-point along a shoreline, appropriate corrections to CPUE, BPUE, density and biomass measures were made (Table S-2). The standardized set area of 3.0 ha allowed simple calculation of density and biomass from CPUE and BPUE data. For estimates of biomass, a correction was made for any missing weights by adding the average weight of turtles in that set.
The CPUE literature suggests that sampling where the target species is most likely to be encountered is problematic and warns against sampling areas that produce “good catches.” We, therefore, established regular sampling at a set of 10 Core sites in the mid-1990s that were sampled with regularity in subsequent years to serve as a baseline of change in the aggregation. Core sites were chosen based on previous sampling history and distribution around the Platform and continued to be sampled even when the study species’ presence declined.
Factors that introduce sampling error to each set of the net include the exact positioning of the set at a sampling site (Fig. 1), speed at which the net was deployed, degree of deviation from a perfect circle, and amount of net overlap (degree of closure). Samples were taken in all months without regard to tidal cycle but were limited to daylight hours between 8AM and 5PM. We attempted to keep track of turtles that escaped the set, were captured on the outside of the net, or were left behind in the set when the netting session was ended, but this was not always possible. We believe that these sources of potential error remained constant over the study. There is no evidence that any of them introduced systematic error into the results. For some analyses, we have used 5-year and 9-year floating averages to reduce variation due to limited annual sampling (e.g., Fig. 2).
Analysis of change in average turtle size and mass and a decadal analysis of aggregation structure used data from the entire study period. Analyses of site-specific changes in CPUE (density), BPUE (biomass), turtle size, and condition index, are limited to sets made between 1992 and 2018. We used linear regression to examine changes in the average size and mass of turtles and restricted these analyses to years with ≥ 40 captures. Non-parametric ANOVA was used to examine changes in the aggregation size structure. Calculation of average size at departure from the Bermuda Platform follows the methods of Meylan et al. (2011) in which it is assumed that the right-hand side of a histogram of aggregation structure (Fig. 3) is determined by departure from the foraging aggregation of individuals belonging to different 5 cm size classes. The proportion of the most numerous size class that is absent from each succeeding size class is assumed to have emigrated and is used to calculate a weighted average for size at departure by decade. Changes in density, biomass, and body condition index (BCI) were examined using linear regression.
For our data set, BCI calculated as (mass.(SCL^3)−1) scaled with SCL (Fig. S-1). To remove the effect of size, we used a residual calculated as the difference between log observed mass and the log mass predicted by the relationship between SCLmin and mass from Meylan et al. (2011: Table 3) (Hayes and Shonkwiler 2001). To test for changes in BCI, an average was calculated for each set of the net made between 1992 and 2018 for which size and mass measurements were available. To examine these data for density-dependent effects on condition index, we plotted average BCI of turtles from all sets at Core sites against total biomass of turtles in that set.
Changes in the distribution (inshore, nearshore, offshore) of the C. mydas aggregation across the Platform were examined by comparing the site-specific biomass for August sets of the net across years pooled by shoreline category (see Table 1 for shoreline categories). If biomass data were not available for a shoreline category for a given year (n = 6), we used an average of the preceding and next measured values.
We used two methods to test for evidence of change in site fidelity. First, all data from 1968 to 2018 were examined to determine the rate at which known individual green turtles moved between named seagrass meadows between captures; only years with 10 or more recaptures were included. Separately, for captures from 1992 to 2018, we used nonparametric ANOVA to test for change in average distance moved by all recaptured turtles between GPS locations of the capture and subsequent recapture sets by year. For both, we used only recaptures where the turtle was released at its previous capture location and not at a different location. We took this precaution because we have observed that moving turtles away from their capture location reduces site fidelity by about 10% (Meylan et al. 2011). For all statistical analyses, results were considered significant at P < 0.05; means are given ± 1 SD unless otherwise noted.
Results
Aggregation—average size and mass
Between 1968 and 2018, the BTP collected data from 5855 green turtle net captures. Between 1 and 288 (114.4 ± 87.6, n = 51) captures were made per year. Sampling with an entrapment net by the research team began in 1975, and by 1976 resulted in adequate sample sizes (n ≥ 40) so that trends in average size (r2 = 0.60, F1,37 = 56.00, P < 0.001, Fig. 2A) and mass (r2 = 0.59, F1,37 = 52.43, P < 0.0001, Fig. 2B) of green turtles in the aggregation can be reported back to that year. Average size and mass declined from 5-year floating averages of 51.6 cm and 23.9 kg in 1978 to 40.1 cm and 13.9 kg in 2016, a decline of 11.5 cm and 13.9 kg. These values represent a 22.3% reduction in average size and a 58.2% reduction in average mass.
Average size (SCLmin) (a) and mass (b) of green turtles (Chelonia mydas) captured on the Bermuda Platform by year, 1976–2018. All net captures from the Bermuda Turtle Project during years with sample sizes ≥ 40 are included. Mean and SE are shown. Linear regressions (dashed lines) are significant (see results). Dotted lines are 5-year floating averages, solid lines are 9-year floating averages. Average annual sample size: carapace length = 147.3 ± 11.9, range 40–299; mass = 142.2 ± 11.4, range 40–283
Aggregation structure and emigration
Analysis of aggregation size structure across five decades revealed significant change (Kruskal–Wallis One-Way Analysis of Variance, H (4) = 642.3, P < 0.001, Fig. 3). All pairwise comparisons between decades differ significantly except 1968–1977 vs. 1988–1997 and 1978–1987 vs. 1998–2007 (Dunn’s Multiple Comparison). Size distribution in early decades is skewed towards larger sizes; those from the last two decades are skewed towards smaller sizes. Through 2017, there was an increase in the number of new recruits (< 30 cm); this size class made up 4.1% of the aggregation in 1968–1977, but 18.4% in 2008–2017. Change in estimated average size at departure is not significant when considered over five decades; however, there is a marked decline from 65.8 cm to 53.5 cm over the last 30 years.
CPUE/density and BPUE/biomass
From 1992 to 2018, average density of green turtles on seagrass meadows increased significantly (Fig. 4) at the 10 Core sites (August only, r2 = 0.42, F1,23 = 16.75, P = 0.0004; all months, r2 = 0.40, F1,25 = 17.00, P = 0.0004) and across all sites (August only, r2 = 0.61, F1,23 = 36.65, P = 0.0001; all months, r2 = 0.66, F1,25 = 47.98, p < 0.0001). Average green turtle density for all samples at all sites was just under two turtles per hectare until about 2005 and increased to about 5 turtles per hectare by 2015. Although density increased significantly over time, biomass did not. For August sets, at the 10 Core sites there was a slightly decreasing trend in biomass (Fig. 5A), at the 15 most-sampled sites, there was a slightly increasing trend (Fig. 5B), but neither was significant. Biomass for August sets changed significantly when examined by shoreline category (Fig. 5B), declining at offshore (r2 = 0.72, F1,25 = 63.74, P < 0.0001) and nearshore (r2 = 0.35, F1,25 = 13.3, P = 0.012) sites, but increasing at inshore sites (r2 = 0.55, F1,25 = 30.34, P < 0.0001). Biomass has been 0 at all offshore sites since 2005 and dropped to zero at nearshore sites in 2014 and 2018. Thus, despite a clear increase in numbers of turtles captured, the decrease in average size and mass of individual turtles resulted in no change in average biomass across either the 10 Core sites or 15 most-sampled sites. Site-specific analyses show that changes in density and biomass on seagrass meadows did not take place in a uniform fashion across the Bermuda Platform (see below).
Average density of green turtles (Chelonia mydas) in seagrass meadows on the Bermuda Platform, 1992–2018. Four categories of sampling are shown. Increase in density for all measures is significant (see results). From 2009 to 2018 all sampling was in August
Annual average biomass (kg ha−1) observed for green turtles (Chelonia mydas) captured on seagrass meadows on the Bermuda Platform. Annual mean and SE are shown for “August” samples at 10 Core sites (a) and at 15 most-sampled sites (b) for 1992–2018. Trend lines are not significant. Averages for offshore sites (solid line) and nearshore sites (dashed line) show significant decline, averages for inshore sites (dotted line) showed significant increase (see results)
Turtle body condition index
Average BCI for all August sets at the 10 Core sites was examined with respect to sample site location: inshore, nearshore, offshore; there was significant decrease in all categories (offshore: r2 = 0.222, F1,62 = 17.71, P < 0.0001; nearshore: r2 = 0.27, F1,71 = 25.84, P < 0.0001; inshore: r2 = 0.11, F1,191 = 24.03, P < 0.0001; Fig. 6). For turtles at offshore sites, BCI decreased at about twice the rate of turtles from inshore sites. Average BCI per set of the net as a function of BPUE revealed a positive relationship (r2 = 0.071, F1,343 = 26.07, P < 0.0001, not shown).
Average body condition index (BCI) by site for green turtles (Chelonia mydas) on the Bermuda Platform, 1992–2018. Data were averaged for “August” sets at Core sites and pooled by shoreline category (see methods). Decline in BCI for all shoreline categories was significant (see results) for offshore sites (triangles, solid line), nearshore sites (squares, dashed line), and inshore sites (circles, dotted line)
Site-specific results
Site-specific results from 1992 to 2018 for density, biomass, turtle size, turtle body condition index and seagrass availability at individual seagrass meadows are given in Table 2. For data pooled for each of 20 sites (Table 1), average green turtle size varied by site from inshore to offshore, with significantly larger turtles found at increasing distance from shore (r2 = 0.30, F1,17 = 7.44, P = 0.0143), and increasing depth (r2 = 0.41, F1,17 = 11.76, P = 0.0032). Change in average turtle size over time varied by shoreline category, with average size decreasing significantly at 4 of 5 offshore and nearshore sites but, with one exception, not changing significantly at inshore sites (Table 2).
Site-specific density and biomass at the 15 most-sampled sites (Table 2) also varied by shoreline category. Both measures decreased at all 5 offshore and nearshore sites (significant at 3 of 5 sites for density, 4 of 5 sites for biomass). However, both measures showed significant increase at 4 of 10 inshore sites, with the remaining inshore sites showing no significant trend. Turtle condition index showed a general pattern of decline across all 15 sites. A significant negative trend in turtle condition index was observed at 7 of the 15 sites (Table 2), including 4 of 5 offshore and nearshore sites (Fig. 6).
Seagrass index
Seagrass index scores showed a general pattern of decline across all 15 sites. Qualitative assessments of seagrass suggest significant decrease at 11 of the 15 sites, including all five of the offshore and nearshore sites. Significant decline in seagrass index score (Fig. 7) was first observed at offshore (r2 = 0.80, F1,17 = 66.41, P < 0.0001), then at nearshore (r2 = 0.81, F1,26 = 113.59, P < 0.0001), and most recently at inshore sites (r2 = 0.52, F1,54 = 57.40, p < 0.0001). We have not observed seagrass at our offshore sites since 2008 or at nearshore sites since 2013 (Fig. 7, Table 2).
Annual average qualitative seagrass index score for 10 Core sampling sites for green turtles (Chelonia mydas) on the Bermuda Platform, 1992–2018, based on records from the project logbook. Declines at offshore sites (triangles, solid line), nearshore sites (squares, dashed line), and inshore sites (circles, dotted line) are significant (see results). See Methods for explanation of shoreline categories. Trends for individual sites are given in Table 2; seagrass index criteria and corresponding percent seagrass coverage are given in Table S-1
Site fidelity of green turtles on the platform
The results of two analyses of site fidelity of green turtles on the Bermuda Platform show similar results. An analysis of movement between named sampling sites since 1979 (Fig. 8A) shows that for 27 sampling years with at least 10 recaptures, an average of 10.6 ± 7.3% (range 0.0 to 26.1%, n = 1317) of recaptured turtles moved between named sampling sites between captures. On average, nearly 90% of recaptures had not switched sites (seagrass meadows). Among those that moved between named sites, most made longshore movements (e.g., nearshore to nearshore site) (7.2% of recaptures), fewer made movements to a shallower site (3.4% of recaptures); very few movements were to a site farther offshore (0.2% of recaptures). By this measure, there appeared to be two periods of reduced site fidelity during which the rate of movement between named sites was about double. An early one occurred during 1992–1997, and a second started in 2011 and continued through 2018.
Measures of site fidelity of green turtles (Chelonia mydas) on the Bermuda Platform. a Proportion of turtles that moved between capture sites between sequential captures (1992–2018). Longshore movement was between offshore sites, between nearshore sites, or between inshore sites; inshore movement was to a shallower site closer to the main islands; offshore movement was to a deeper site farther from the main islands. Results are based on 1287 recaptures. b Average distance (m) moved between captures for all recaptures 1992–2018. Distances were measured between center points of consecutive net sets in which an individual was captured. Distance was significantly greater within peaks centered on 2002 and 2018, relative to low values centered on 2008 (Pairwise Multiple Comparison Procedure; Dunn's Method)
A second analysis of site fidelity using GPS locations for individual sets of the net for the period 1992–2018 (Fig. 8B) shows two peaks of reduced site fidelity that include years with significantly increased mobility relative to years in which the average distance moved was just greater than the diameter of our net sets (195 m) (Kruskal–Wallis One-Way Analysis of Variance, H (26) = 120.4, P < 0.001). Dunn’s Multiple Comparisons confirmed significant differences in average distance moved during most years in two periods, 1996–2004 and 2015–2018, relative to the years 2008–2011 (Fig. 8B). The yellow polygons in Fig. 1 show a rough outline for each of the sampled seagrass meadows. All are quite limited in area so that movements of 1–2 km (Fig. 8B) would also represent a movement between named foraging areas (Fig. 8A).
Discussion
We have documented a series of significant demographic changes in the foraging aggregation of green turtles on the Bermuda Platform, most of which are consistent with expected responses of a population (aggregation) to a decline in resources. The aggregation is highly dynamic with individuals in the smallest size classes recruiting, and those from larger size classes emigrating every year. While on the Platform, all members of the aggregation share a limited set of resources and, as seagrass resources have declined, the aggregation has responded accordingly. Because Bermuda serves only as benthic developmental habitat, and green turtles on the Platform are not mature, reducing reproductive output is not an option for the aggregation to respond to declining resources. Predictable changes might include reduced successful transition from oceanic to benthic stages, departure at smaller size, reduced density and biomass, reduced relative body mass (body condition index), increased foraging effort (as indicated by reduced site fidelity), lower growth rates, a change of diet, and higher mortality rates. Observations reported here allow us to comment on most of these expected changes, but others have been and will continue to be examined separately. A reduction in growth rate has been documented for Bermuda green turtles as part of a growth meta-analysis for this species in the Northwest Atlantic (Bjorndal et al. 2017), which included 845 growth intervals from Bermuda. Temporal change in diet over recent years (2015–2019) has been assessed and found to be minimal (Gulick et al. 2021b). Reduction in survivorship at recruitment to the Bermuda Platform will be examined in a separate study, using data from the Bermuda sea turtle stranding network.
External factors
Some changes in the Bermuda aggregation reported here are likely to be independent of events in Bermuda and instead reflect external factors, specifically, conservation work at nesting beaches in the greater West Atlantic, and a worldwide pattern of seagrass decline. Multiple source rookeries known to contribute to the Bermuda aggregation have shown increasing numbers of nesting females and/or nests (Chaloupka et al. 2008; Seminoff et al. 2015; Mazaris et al. 2017). Immigration into developmental aggregations in the Atlantic has increased at multiple sites (Witherington et al. 2006; Redfoot and Ehrhart 2013; Silva et al. 2017; Howell and Shaver 2021; this study), and is best explained by increased output at recovering rookeries. Seagrass meadows in Bermuda that showed significant increase in density were all inshore sites that historically had the smallest turtles (Table 2), and the increase in density is best explained as the result of increasing numbers of new recruits in the 25–35 cm size range (Fig. 3) coming from recovering rookeries.
The decline in the seagrass resource base for green turtles that we corroborate for Bermuda (see also Murdoch et al. 2007; Fourqurean et al. 2010, 2019; Manuel et al. 2013) is part of a worldwide trend. A review of the status of seagrasses (Waycott et al. 2009) suggests that decreases in the health of seagrass ecosystems were detected well before the relatively recent progress towards recovery of some green turtle populations. A steady increase in reports of decline in seagrass ecosystems was traced back to the 1930’s (Waycott et al. 2009), decades before major conservation efforts for green turtles began on nesting beaches in the Atlantic. Population Viability Analyses from Seminoff et al. (2015) indicate an increasing trend in nesting females at four major source areas for Bermuda, starting in 1970 or later; increases can be traced back to about 1970 at Tortuguero, Costa Rica, and back to about 1990 at sites in Cuba, Mexico and Florida.
Aggregation structure and earlier emigration
The average size of green turtles on foraging grounds in Bermuda decreased by about one-quarter and average mass by more than half over the course of our study. The size at which recruits appear has remained constant, but the relative number of turtles in the smallest size classes has increased. Thus, the expectation that there should be less successful transition to a benthic life stage (i.e., fewer small turtles) was not met. Reduction in larger size classes indicates that our expectation of smaller size at departure was met. Departure from Bermuda, involving a developmental migration to a more tropical foraging area, is expected for members of this aggregation (Carr et al. 1978; Meylan et al. 2011). Estimated size at departure declined by more than 10 cm during the last three decades of the study. Recent observations of green turtles tagged in Bermuda and recaptured alive on the east coast of Florida (M8149 and MB2248) indicate that green turtles as small as 42.7 and 51.0 cm SCLmin can make a successful open-ocean migration from Bermuda to a foraging area 1500 + km distant (BTP unpubl data).
Growth rates of green turtles in the West Atlantic, including those in Bermuda, declined on average by 26% between 1999 and 2015 (Bjorndal et al. 2017). For larger turtles, a reduction in growth rate could be the cue to emigrate. Previously, we suggested that green turtles departed Bermuda when they reached puberty (detected by laparoscopy), and that hormonal changes associated with the onset of puberty might trigger this migration (Meylan et al. 2011). More recently, Bjorndal et al. (2019), suggested that green turtles depart their developmental site if resources are insufficient to maintain adequate growth. They detected a decline in the variance of growth rate for large immatures in the Bahamas which they attributed to the departure of slow-growing individuals. This new model offers a better explanation for the reduction in size at departure from Bermuda. The case for reduced growth rate as the cue, rather than the onset of maturity, is supported by our observation that very few green turtles in Bermuda begin maturation at a size of 60 cm or less (Meylan et al. 2011).
The marked reduction in average size and mass of individual turtles is consistent with expectations drawn from a recent stable isotope (SI) study of green turtle diet done in conjunction with BTP sampling in 2012 and 2013 (Burgett et al. 2018). That study estimated that seagrass contribution to the tissues of green turtles varied from 5–80% (average 47%) with an ontogenetic shift to a more seagrass-focused diet at a size of ~ 40 cm. Turtles over 50 cm were suggested to have seagrass-dominated diets, although SI data suggested that they still appeared to use other resources. This reconstruction of diet from SI data was not consistent with results reported by a recent analysis of gut contents (Gulick et al. 2021b), which found seagrasses to dominate the diet across all size classes. These two studies measured diet differently, i.e., what is assimilated vs. what is ingested. The inconsistency between them likely reflects the rate of conversion of diet components to tissue and the rate of turnover of tissues (Seminoff et al. 2006), perhaps in combination with the reduced digestive efficiency of T. testudinum in relatively small green turtles (Bjorndal 1980). Despite the different results of the SI and gut sample studies, given the increased rate of intake (Bjorndal 1980) and reliance on seagrass (Burgett et al. 2018) by larger turtles, our documented change in the aggregation indicates a significant reduction in the larger turtles that consume more seagrass.
Changes in density and biomass
With declining seagrass resource availability, we expected to find a reduction in green turtle density and biomass. However, across all sites combined, average density more than doubled and biomass showed no significant change over the study period. Significant increase in site-specific density and biomass occurred only at 4 of 10 inshore sites with turtle density and biomass decreasing significantly at most offshore and nearshore sites (Table 2). These 4 inshore sites were among the 5 sites with the smallest average size turtles, and none had a significant increase in average turtle size, indicating that increased density and biomass at these sites resulted from an increase in the number of recruits rather than movement of large turtles inshore. The highest density measured in Bermuda was at Baileys Bay, the site with the smallest average turtle size (Table 2). Density was 37.2 turtles ha−1 at this site on one occasion in August 2017. This single sample was a clear outlier with the next highest density measured at 23.2 turtles ha−1, and fewer than 3% of samples (n = 739) having a density above 10 turtles ha−1. The average density for all sets in Bermuda remained below 2 turtles ha−1 until 2005, and then increased to 5–6 turtles ha−1 in recent years (Fig. 4). No increase in average green turtle density occurred prior to 2004, when the onset of seagrass decline was first documented (Murdoch et al. 2007). Average density in Bermuda was always well below the carrying capacity observed on seagrass meadows at a more tropical benthic developmental site, Union Creek, Inagua, Bahamas, of about 10 turtles ha−1 (Bjorndal et al. 2000), although carrying capacity at Bermuda’s more temperate location might be expected to be lower.
Because large green turtles consume much more seagrass than smaller ones (Bjorndal 1980) turtle biomass is more useful than density alone as a measure of grazing impact on seagrass meadows (Scott et al. 2018). Turtle biomass in Bermuda exceeded 200 kg ha−1 in fewer than 1% of samples and exceeded 100 kg ha−1 in only 8.4% of samples. Reports of biomass on seagrass meadows at other sites around the globe suggest that values for C. mydas in Bermuda (average 14.7 to 62.3 kg ha−1 for the 15 most-sampled sites) are relatively low. Bjorndal et al. (2000) suggested a carrying capacity in Inagua of about 185 kg ha−1. Moran and Bjorndal (2005) provided estimates of carrying capacity using three levels of intake that ranged from 122 to 292 kg ha−1 under conditions of heavy grazing. Cardona et al. (2020) reported on sites at Fernando de Noronha (Brazil), and Hawaii, at which C. mydas biomass was estimated as more than 500 kg ha−1 to more than 1000 kg ha−1. Sites in the Indian Ocean reported to have densities ranging from 10 turtles ha−1 (Lakshadweeps, India; Kelkar et al 2013) to 24 turtles ha−1 (Mayotte, Ballorain et al. 2010) support both large juveniles and adults and could also have biomass in the 500–1000 kg ha−1 range.
Greater foraging effort
Given the known site fidelity to specific foraging areas exhibited by green turtles in Bermuda (Meylan et al. 2011), increased movement between known foraging areas could be interpreted as increased foraging effort. Alternatively, reduced fidelity could result from increased disturbance, such as increased vessel traffic. Each of the two methods we used to test for changes in site fidelity showed two peaks of reduced fidelity (Fig. 8). The first occurred around the turn of the millennium, the second occurred in the last decade. Given this bimodal pattern (impact of vessel traffic would likely be linear), we interpret the reduced site fidelity as evidence that turtles moved more frequently and greater distances to meet foraging requirements during these two periods. The first began just before the period when a significant reduction in growth rates occurred for three different sea turtle species in the West Atlantic, including green turtles (Bjorndal et al. 2017). The second likely reflects the decline in seagrass habitat across the Bermuda Platform that has been well documented over the last two decades.
Offshore to inshore pattern
Seagrass productivity is known to decrease with increasing water depth and reduced light availability (Tomasko and Dawes 1990) and increased depth would exacerbate any increase in turbidity and/or colder water temperatures. Depth was found to be a significant regulating factor in compensatory growth in T. testudinum in the USVI (Gulick et al. 2020, 2021a). Furthermore, larger turtles, with the capacity to consume more seagrass are found farther offshore and in deeper water (Tables 1 and 2). These factors explain why our site-specific results support an offshore to inshore pattern of seagrass decline as reported by Murdoch et al. (2007) and Fourqurean et al. (2019). As this decline in seagrass resource occurred, a decrease in average turtle size was observed at offshore and nearshore sites, and density and biomass declined significantly at most of them (Table 2). However, no mass influx of larger turtles into nearshore sites was documented (Fig. 8A); there was an increase in average size at only 1 of 10 inshore sites (Table 2). At four of five offshore and nearshore sites, where the largest turtles were found, a significant reduction in turtle size was detected before turtles disappeared from these sites (Table 2). No inshore sites showed a significant decrease in turtle size.
In addition, BCI declined significantly, with a clear offshore to inshore pattern (Fig. 6), occurring earlier and more rapidly offshore and then later at nearshore and inshore sites. The index used here is easily understood as 0.0 represents the expected mass of an individual of a specific size (based on data collected from 2473 C. mydas in Bermuda, 1968–2005); positive values are turtles that are heavier than expected, negative values are those not as heavy as expected. To put these values in perspective, a sample of 37 green turtles (21.4–45.5 cm) documented by the Bermuda Sea Turtle Stranding Network as “thin or emaciated”, had an average BCI of -0.159 ± 0.052 (range -0.054 to -0.248). During the last decade, turtles from the 10 Core sites had average BCI in the -0.02 to -0.09 range with about 15% of captures falling in this “emaciated” range.
Grazing and synergistic stressors
Relative to mammalian megaherbivores, green turtles have very small metabolic requirements (Bjorndal 1980); they were reported to ingest remarkably small amounts daily (0.24–0.33% of body mass, dry weight to wet weight). Using this estimate, a 20 kg green turtle would consume between 44 and 66 gm (dry weight) of seagrass per day. Given this low rate of intake and the relatively small size of individual turtles and low total biomass of green turtles on seagrass meadows in Bermuda, healthy seagrass meadows should have been able to withstand green turtle grazing pressure (Moran and Bjorndal 2005; Fourqurean et al. 2010). Average turtle biomass across all sampling sites remained constant over the course of our study and average turtle size decreased significantly. Because smaller turtles are less reliant on seagrass than larger ones (Burgett et al. 2018), grazing pressure on seagrasses should have decreased over the last 50 years as a result of decreasing turtle size.
Under certain circumstances, seagrasses in Bermuda can survive many years of repeated grazing (Fourqurean et al. 2010). Using experimental exclusion, these authors found that ungrazed areas had greater levels of aboveground biomass when compared to grazed areas, whereas there was no difference in shoot density or belowground biomass (i.e., roots and rhizomes). However, that study suggested that high rates of grazing caused declines in rhizome soluble carbohydrate that, in turn, reduced the ability of T. testudinum to recover from unfavorable environmental conditions. Work at their Chub Head Beacon site agreed with results of other studies that have suggested that healthy seagrass meadows should be resilient to repeated grazing (i.e., Moran and Bjorndal 2005; Gulick et al. 2020, 2021a), and they concluded that some other environmental factor was important in determining when seagrasses can survive heavy grazing pressure in Bermuda. This idea is consistent with work done in Australia (Fraser et al. 2014; Kendrick et al. 2019) that suggests that “synergistic stressors” resulted in the loss of seagrass meadows there.
Synergistic stressors have been previously identified as a cause of seagrass decline in the West Atlantic. Williams (1988) reported that the combination of anchor chains and grazing led to decline of seagrass meadows in the US Virgin Islands. Murdoch et al. (2007) identified cool winter temperatures, short day length at 32oN, and low nutrient availability as possible sources of reduced productivity and additional sources of stress on seagrasses in Bermuda. Fraser et al. (2014) concluded that “stressors are unlikely to act singly in natural ecosystems, especially during extreme climatic events”. These authors emphasized the susceptibility of seagrasses at the edge of their range to climatic events and suggested that multiple synergistic stressors may be responsible in such cases. We suggest that temperature extremes, bioturbation, and P limitation are stressors that may have acted in synergy with grazing in Bermuda.
Studies of temperature impacts on seagrass have understandably focused on increase in temperature (e.g., Rasheed and Unsworth 2011; Arias-Ortiz et al. 2018), especially in shallow water (Campbell et al. 2006). However, impacts in Bermuda clearly began at the deepest sites (Murdoch et al. 2007; Fourqurean et al. 2019; this study) which would be inconsistent with high temperature stress. In fact, Murdoch et al. (2007) identified cool winter temperatures and short day-length at 32oN as possible sources of reduced seagrass productivity in Bermuda. They specifically identified a wintertime cold-water event related to the North Atlantic Oscillation that occurred in 1996 as a possible cause of below-normal productivity in seagrasses. Zieman (1975) summarized early studies of seagrass productivity and reported that T. testudinum prefers temperatures of 20–30 °C; productivity was said to drop to near zero at temperatures below 19 °C. Lee et al. (2007) reported that optimal temperatures for T. testudinum productivity range from 28 °C to 31 °C. A summary of water temperatures in Bermuda recorded at NOAA National Data Buoy Center station (BEPB6-2,695,540), shows that temperatures fell below 20 °C annually for at least 3 months (Jan.–March) during the period 2009–2019 and were optimal for T. testudinum growth only during July–September. During winters of 1995–1998, temperatures at this station remained below 20 °C from at least mid-December to mid-April (4 months), and in the winter of 1997, from 28 November to 1 May (5 months). Long-term cold stress would be consistent with the decline in seagrasses observed in Bermuda that started at the deepest sites by 2000 (Fig. 7). Cold temperatures would limit the ability of T. testudinum to maintain a critical minimum of leaf tissue to withstand prolonged grazing via compensatory growth (Gulick et al. 2021a). Recent, climate-induced, “tropicalization” (Rodriguez and Heck 2020; Valentine and Heck 2021) is not a likely explanation for seagrass loss in Bermuda which has a long history of supporting a tropical fauna, including significant green turtle biomass (Babcock 1938).
Bioturbation can be an additional stress on seagrass (DeWitt 2009; Fourqurean et al. 2010). Kelkar et al. (2013) suggested that grazing pressure may potentially change sediment dynamics and impact water quality. Because turtles do not typically dig up seagrass, bioturbation caused directly by green turtles is not likely to be an issue. Multiple authors have contrasted green turtles with sirenians, pointing out that the latter excavate rhizomes, while the former usually do not (Lanyon et al. 1989). Rhizomes are not a regular part of the green turtle diet (Bjorndal 1980; Mortimer 1981) and they were relatively rare in gut samples from Bermuda (Gulick et al. 2021b). Johnson et al. (2019) suggested that the rhizome mats of T. testudinum are deeper and more robust than those of species reported to be dug up by sea turtles in Indonesia (Christianen et al. 2014), making this an unlikely strategy for green turtles in the Caribbean. Rays can be an additional source of bioturbation on seagrass meadows (DeWitt 2009). Observations in the BTP logbook include numerous accounts of “blowouts” or “potholes” (at least 48 records) in seagrass meadows that were consistent with feeding by rays. In some cases, an entire sample site was covered in “potholes”. Spotted eagle rays are abundant in Bermuda (Ajemian et al. 2012) and one of the most common forms of bycatch during turtle sampling (at least 52 records). There has been concern expressed about the impact of large numbers of rays on the molluscan fauna of Bermuda (Ajemian et al. 2012), but the impact of rays on seagrass meadows has not been assessed.
Phosphorus (P) limitation is a critical consideration for the productivity of seagrasses in Bermuda (Murdoch et al. 2007; Holzer and McGlathery 2016; Fourqurean et al. 2015). Experimental work undertaken in one of our regularly sampled sites (Baileys Bay) suggests that, during the period 2007–2009, response by T. testudinum to simulated grazing was P limited (Holzer and McGlathery 2016). Areas of the Platform from which seagrasses disappeared first were those with the largest C. mydas, most likely to suffer from long-term cold stress, and the greatest P limitation (Fourqurean et al. 2015). Furthermore, a possible role for marine turtles in the P cycle has been overlooked. Sea turtles have the capacity to move large amounts of nutrient and energy between ecosystems over a large geographic range (Lanyon et al. 1989; Bouchard and Bjorndal 2000). The nutrients removed from benthic developmental sites from which large immature sea turtles emigrate could be substantial. P is a critical element in vertebrate bone (CaPO4) formation. Green turtles arrive in Bermuda weighing just over 1 kg and depart weighing 40–60 kg (reduced to 30–50 kg in recent years) and all resources used in growth and maintenance are derived from ecosystems on the Platform. The turtle skeleton makes up roughly 20% of its mass (Iverson 1984) and turtle bone is about 10% P (Biltz and Pelligrino 1969). A 50 kg turtle would remove about 1 kg of P when it departed the Platform making it unavailable for recycling through the detritus pathway. Removal of significant amounts of P by C. mydas over a long timeframe could exacerbate this well-established limitation on seagrass growth.
Conclusions
Ecological equilibrium models predict that demographic responses should take place in predator populations in response to reduction in available prey. We believe this has occurred in the Bermuda green turtle aggregation over at least the last three decades, if not longer. The role of Bermuda in the life cycle of green turtles in the greater West Atlantic and Caribbean has changed. In the 1990s, immature green turtles belonging to multiple genetic populations could complete nearly all of their benthic developmental sequence at this site. At present, only a portion of that stage can be completed. Previously, C. mydas would emigrate from Bermuda at stage 2 (pubescent) and then complete maturation elsewhere (Meylan et al. 2011). However, due to seagrass loss they now depart years before they begin the maturation process and instead will spend additional time on foraging grounds elsewhere before maturing. The composition of the aggregation was already changing when biologists first perceived the issue of seagrass loss; it began with a steady decline in average size and mass of turtles (at least since 1976) that has been continuous. Decline in average size suggests progressively earlier emigration that is advantageous for both sea turtles and seagrass. Larger green turtles in Bermuda show a higher reliance on seagrass rather than other food resources and can be expected to consume more on a daily basis than small turtles (Bjorndal 1980). At smaller sizes, green turtles may be able to rely on macroalgae and animal foods for more than 50% of their nutrients (Burgett et al. 2018), but there appears to be variation in the reliance of small immatures on seagrasses at local scales (Howell et al. 2016; Howell and Shaver 2021). Our work suggests that the “dynamic view of seagrass meadows” (Christianen et al. 2021) must take into account the dynamics of the green turtle populations/aggregations that use them, as well as the role of synergistic stressors.
Given the wide range of stressors that impact seagrasses, especially at the edge of their geographic range, the green turtle aggregation in Bermuda cannot be confidently singled out as a single cause for the loss of seagrasses; a combination of synergistic stressors is more likely (Murdoch et al. 2007; Fraser et al. 2014). The causes of seagrass decline in Bermuda cannot be determined until all sources of stress are identified and the changing role of C. mydas should be kept in perspective. Our results show that studies of the interactions between green turtles and seagrasses should not rely solely on simple density measures of sea turtles (e.g., Lal et al. 2010; Kelkar et al. 2013), but rather, biomass and size-class interactions with seagrasses must be considered (Burgett et al. 2018; Scott et al. 2018). It has been suggested that there is little evidence that turtles in Bermuda are responding to the loss of their primary food (Fourqurean et al. 2019). However, we have been able to show significant demographic changes that started before seagrass decline was detected; the changes detected should have significantly reduced the impact of grazing on seagrasses in Bermuda.
Bjorndal et al. (2005) have suggested that “monitoring [of sea turtles] should be continued as long as there are management issues.” They argue that long-term studies (like the one in Bermuda) are important for assessing trends. Esteban et al. (2020) have recently summarized the evidence for flexibility in the C. mydas diet. They have shown that there is more use of animal matter at sites with cooler temperatures, including sites at the edge of the range. This flexibility may enable adaptation to changing resource patterns. In Bermuda, cold water temperatures in cold winters and continuous grazing during warm winters, combined with P limitation, are the most likely synergistic stressors that have led to the inability of seagrasses in Bermuda to withstand constant grazing pressure by a species on the road to recovery.
Data availability
The dataset analyzed during the current study will be provided upon reasonable request submitted to the corresponding author.
References
Ajemian MJ, Powers SP, Murdoch TJ (2012) Estimating the potential impacts of large mesopredators on benthic resources: integrative assessment of spotted eagle ray foraging ecology in Bermuda. PLOS One 7:e40227. https://doi.org/10.1371/journal.pone.0040227
Arias-Ortiz A, Serrano O, Masqué P, Lavery PS, Mueller U, Kendrick GA, Rozaimi M, Esteban A, Fourqurean JW, Marbà NJNCC, Mateo MA (2018) A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nat Clim Change 8:338–344. https://doi.org/10.1038/s41558-018-0096-y
Babcock HL (1938) The sea-turtles of the Bermuda Islands, with a survey of the present state of the turtle fishing industry. Proc Zool Soc Lond 7:595–602. https://doi.org/10.1111/j.1469-7998.1938.tb00015.x
Ballorain K, Ciccione S, Bourjea J, Grizel H, Enstipp M, Georges JY (2010) Habitat use of a multispecific seagrass meadow by green turtles Chelonia mydas at Mayotte island. Mar Biol 157:2581–2590. https://doi.org/10.1007/s00227-010-1520-7
Bass AL, Witzell WN (2000) Demographic composition of immature green turtles (Chelonia mydas) from the east central Florida coast: evidence from mtDNA markers. Herpetologica 56:357–367
Biltz RM, Pellegrino ED (1969) The chemical anatomy of bone. J Bone Joint Surg 51A:456–466. https://doi.org/10.2106/00004623-196951030-00003
Bjorndal KA (1979) Cellulose digestion and volatile fatty acid production in the green turtle, Chelonia mydas. Comp Biochem Physiol A 63:127–133. https://doi.org/10.1016/0300-9629(79)90638-8
Bjorndal KA (1980) Nutrition and grazing behavior of the green turtle Chelonia mydas. Mar Biol 56:147–154. https://doi.org/10.1007/BF00397131
Bjorndal KA (1982) The consequences of herbivory for the life history pattern of the Caribbean green turtle, Chelonia mydas. In: Bjorndal KA (ed) Biology and conservation of sea turtles. Smithsonian Institution Press, Washington, DC, pp 111–116
Bjorndal KA (1985) Nutritional ecology of sea turtles. Copeia 1985:736–751. https://doi.org/10.2307/1444767
Bjorndal KA (1997) Foraging ecology and nutrition of sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles, vol 1. CRC Press. Boca Raton, FL, pp 199–231
Bjorndal KA, Bolten AB, Chaloupka MY (2000) Green turtle somatic growth model: evidence for density dependence. Ecol Appl 10:269–282. https://doi.org/10.1890/1051-0761(2000)010%5B0269:GTSGME%5D2.0.CO;2
Bjorndal KA, Bolten AB, Chaloupka MY (2005) Evaluating trends in abundance of immature green turtles, Chelonia mydas, in the greater Caribbean. Ecol Appl 15:304–314. https://doi.org/10.1890/04-0059
Bjorndal KA, Bolten AB, Chaloupka M, Saba V, Bellini C, Marcovaldi MA, Santos AJ, Bortolon LF, Meylan AB, Meylan PA, Gray J et al (2017) Ecological regime shift drives declining growth rates of sea turtles throughout the West Atlantic. Glob Chang Biol 23:4556–4568. https://doi.org/10.1111/gcb.13712
Bjorndal KA, Bolten AB, Chaloupka M (2019) Green turtle somatic growth dynamics: distributional regression reveals effects of differential emigration. Mar Ecol Prog Ser 616:185–195. https://doi.org/10.3354/meps12946
Bolten AB (1999) Techniques for measuring sea turtles. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA, Donnelly M (eds) Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group Publication No. 4, pp 110–114
Bouchard SS, Bjorndal KA (2000) Sea turtles as biological transporters of nutrients and energy from marine to terrestrial ecosystems. Ecology 81:2305–2313. https://doi.org/10.1890/0012-9658(2000)081%5B2305:STABTO%5D2.0.CO;2
Brand-Gardner SJ, Limpus CJ, Lanyon JM (1999) Diet selection by immature green turtles, Chelonia mydas, in subtropical Moreton Bay, south-east Queensland. Aust J Zool 47:181–191. https://doi.org/10.1071/ZO98065
Burgett CM, Burkholder DA, Coates KA, Fourqurean VL, Kenworthy WJ, Manuel SA, Outerbridge ME, Fourqurean JW (2018) Ontogenetic diet shifts of green sea turtles (Chelonia mydas) in a mid-ocean developmental habitat. Mar Biol 165:1–12. https://doi.org/10.1007/s00227-018-3290-6
Campbell SJ, McKenzie LJ, Kerville SP (2006) Photosynthetic responses of seven tropical seagrasses to elevated seawater temperature. J Exp Mar Biol Ecol 330:455–468. https://doi.org/10.1016/j.jembe.2005.09.017
Cardona L, Campos P, Velásquez-Vacca A (2020) Contribution of green turtles Chelonia mydas to total herbivore biomass in shallow tropical reefs of oceanic islands. PLOS One 15:e0228548. https://doi.org/10.1371/journal.pone.0228548
Carr AF, Carr MH, Meylan AB (1978) The ecology and migrations of sea turtles 7. The West Caribbean green turtle colony. Bull Am Mus Nat Hist 162:1–46
Chaloupka M, Bjorndal KA, Balazs GH, Bolten AB, Ehrhart LM, Limpus CJ, Suganuma H, Troëng S, Yamaguchi M (2008) Encouraging outlook for recovery of a once severely exploited marine megaherbivore. Glob Ecol Biogeogr 17:297–304. https://doi.org/10.1111/j.1466-8238.2007.00367.x
Christianen MJ, Herman PM, Bouma TJ, Lamers LP, van Katwijk MM, van der Heide T, Mumby PJ, Silliman BR, Engelhard SL, van de Kerk M, Kiswara W (2014) Habitat collapse due to overgrazing threatens turtle conservation in marine protected areas. Proc R Soc Lond B 281:20132890. https://doi.org/10.1098/rspb.2013.2890
Christianen MJ, van Katwijk MM, van Tussenbroek BI, Pagès JF, Ballorain K, Kelkar N, Arthur R, Alcoverro T (2021) A dynamic view of seagrass meadows in the wake of successful green turtle conservation. Nat Ecol Evol 5:553–555. https://doi.org/10.1038/s41559-021-01433-z
De Laubenfels MW (1950) An ecological discussion of the sponges of Bermuda. Trans Zool Soc Lond 27:155–201. https://doi.org/10.1111/j.1096-3642.1950.tb00228.x
DeWitt TH (2009) The effects of bioturbation and bioirrigation on seagrasses. Seagrasses and protective criteria: a review and assessment of research status. Office of Research and Development, National Health and Environmental Effects Research Laboratory, EPA/600/R-09/050
Dodd CK, Morgan GS (1992) Fossil sea turtles from the early Pliocene bone valley formation, central Florida. J Herpetol 26:1–8. https://doi.org/10.2307/1565014
Esteban N, Mortimer JA, Stokes HJ, Laloë JO, Unsworth RK, Hays GC (2020) A global review of green turtle diet: sea surface temperature as a potential driver of omnivory levels. Mar Biol 167:1–17. https://doi.org/10.1007/s00227-020-03786-8
Fourqurean JW, Manuel S, Coates CA, Kenworthy WJ, Smith SR (2010) Effects of excluding sea turtle herbivores from a seagrass bed: overgrazing may have led to loss of seagrass meadows in Bermuda. Mar Ecol Prog Ser 419:223–232. https://doi.org/10.3354/meps08853
Fourqurean JW, Manuel SA, Coates KA, Kenworthy WJ, Boyer JN (2015) Water quality, isoscapes and stoichioscapes of seagrasses indicate general P limitation and unique N cycling in shallow water benthos of Bermuda. Biogeosciences 12:6235–6249. https://doi.org/10.5194/bg-12-6235-2015
Fourqurean JW, Manuel SA, Coates KA, Massey SC, Kenworthy WJ (2019) Decadal monitoring in Bermuda shows a widespread loss of seagrasses attributable to overgrazing by the green sea turtle Chelonia mydas. Estuar Coasts 42:1524–1540. https://doi.org/10.1007/s12237-019-00587-1
Fraser MW, Kendrick GA, Statton J, Hovey RK, Zavala-Perez A, Walker DI (2014) Extreme climate events lower resilience of foundation seagrass at edge of biogeographical range. J Ecol 102:1528–1536. https://doi.org/10.1111/1365-2745.12300
Grech A, Chartrand-Miller K, Erftemeijer P, Fonseca M, McKenzie L, Rasheed M, Taylor H, Coles R (2012) A comparison of threats, vulnerabilities and management approaches in global seagrass bioregions. Environ Res Lett 7:024006. https://doi.org/10.1088/1748-9326/7/2/024006
Gulick AG, Johnson RA, Pollock CG, Hillis-Starr Z, Bolten AB, Bjorndal KA (2020) Recovery of a large herbivore changes regulation of seagrass productivity in a naturally grazed Caribbean ecosystem. Ecology 101:e03180. https://doi.org/10.1002/ecy.3180
Gulick AG, Johnson RA, Pollock CG, Hillis-Starr Z, Bolten AB, Bjorndal KA (2021a) Recovery of a cultivation grazer: a mechanism for compensatory growth of Thalassia testudinum in a Caribbean seagrass meadow grazed by green turtles. J Ecol 109:3031–3045. https://doi.org/10.1111/1365-2745.13718
Gulick AG, Meylan AB, Meylan PA, Hart KM, Gray JA, Roth G, Bolten AB, Bjorndal KA (2021) Role of ingesta particle size in the green turtle grazing strategy, ontogenetic diet shifts, and responses to seagrass declines. Mar Bio 168:1–14. https://doi.org/10.1007/s00227-021-03965-1
Hardy RF, Hu C, Witherington B, Lapointe B, Meylan A, Peebles E, Meirose L, Hirama S (2018) Characterizing a sea turtle developmental habitat using landsat observations of surface-pelagic drift communities in the eastern Gulf of Mexico. IEEE J Sel Top Appl Earth Obs Remote Sens 11:3646–3659. https://doi.org/10.1109/JSTARS.2018.2863194
Hayes JP, Shonkwiler JS (2001) Morphometric indicators of body condition: worthwhile or wishful thinking? In: Speakman JR, Hayes JP, Shonkwiler JS (eds) Body composition analyses of animals: a handbook of non-destructive methods. Cambridge University Press, pp 8–38
Holzer KK, McGlathery KJ (2016) Cultivation grazing response in seagrass may depend on phosphorus availability. Mar Biol 163:88. https://doi.org/10.1007/s00227-016-2855-5
Howell LN, Shaver DJ (2021) Foraging habits of green sea turtles (Chelonia mydas) in the northwestern Gulf of Mexico. Front Mar Sci 8:658368. https://doi.org/10.3389/fmars.2021.658368
Howell LN, Reich KJ, Shaver DJ, Landry AM, Gorga C (2016) Ontogenetic shifts in diet and habitat of juvenile green sea turtles in the northwestern Gulf of Mexico. Mar Ecol Prog Ser 559:217–229. https://doi.org/10.3354/meps11897
Ivany LC, Portell RW, Jones DS (1990) Animal-plant relationships and paleobiogeography of an Eocene seagrass community from Florida. Palaios 5:244–258. https://doi.org/10.2307/3514943
Iverson JB (1984) Proportional skeletal mass in turtles. Florida Sci 47:1–11
Johnson RA, Gulick AG, Bolten AB, Bjorndal KA (2019) Rates of sediment resuspension and erosion following green turtle grazing in a shallow Caribbean Thalassia testudinum meadow. Ecosystems 22:1787–1802. https://doi.org/10.1007/s10021-019-00372-y
Kelkar N, Arthur R, Marbà N, Alcoverro T (2013) Greener pastures? High-density feeding aggregations of green turtles precipitate species shifts in seagrass meadows. J Ecol 101:1158–1168. https://doi.org/10.1111/1365-2745.12122
Kendrick GA, Nowicki RJ, Olsen YS, Strydom S, Fraser MW, Sinclair EA, Statton J, Hovey RK, Thomson JA, Burkholder DA, McMahon KM (2019) A systematic review of how multiple stressors from an extreme event drove ecosystem-wide loss of resilience in an iconic seagrass community. Front Mar Sci 6:455. https://doi.org/10.3389/fmars.2019.00455
Lahanas PN, Bjorndal KA, Bolten AB, Encalada SE, Miyamoto MM, Valverde RA, Bowen BW (1998) Genetic composition of a green turtle (Chelonia mydas) feeding ground population: evidence for multiple origins. Mar Biol 130:345–352. https://doi.org/10.1007/s002270050254
Lal A, Arthur R, Marbà N, Lill AW, Alcoverro T (2010) Implications of conserving an ecosystem modifier: increasing green turtle (Chelonia mydas) densities substantially alters seagrass meadows. Biol Conserv 143:2730–2738. https://doi.org/10.1016/j.biocon.2010.07.020
Lanyon JM, Limpus C, Marsh H (1989) Dugongs and turtles: grazers in the seagrass system. In: Larkum AWD, Mccomb AJ, Shepherd SA (eds) Biology of seagrasses. A treatise on the biology of seagrasses with special reference to the Australian region. Aquatic plant studies. Elsevier, Amsterdam, pp 610–634
Lee KS, Park SR, Kim YK (2007) Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: a review. J Exp Mar Biol Ecol 350:144–175
Lemons G, Lewison R, Komoroske L, Gaos A, Lai CT, Dutton P, Eguchi T, LeRoux R, Seminoff JA (2011) Trophic ecology of green sea turtles in a highly urbanized bay: insights from stable isotopes and mixing models. J Exp Mar Biol Ecol 405:25–32. https://doi.org/10.1016/j.jembe.2011.05.012
Luke K, Horrocks JA, LeRoux RA, Dutton PH (2004) Origins of green turtle (Chelonia mydas) feeding aggregations around Barbados, West Indies. Mar Biol 144:799–805. https://doi.org/10.1007/s00227-003-1241-2
Mansfield KL, Wyneken J, Luo J (2021) First Atlantic satellite tracks of ‘lost years’ green turtles support the importance of the Sargasso sea as a sea turtle nursery. Proc R Soc Lond B 288:20210057. https://doi.org/10.1098/rspb.2021.0057
Manuel SA, Coates KA, Kenworthy WJ, Fourqurean JW (2013) Tropical species at the northern limit of their range: composition and distribution in Bermuda’s benthic habitats in relation to depth and light availability. Mar Environ Res 89:63–75. https://doi.org/10.1016/j.marenvres.2013.05.003
Mazaris AD, Schofield G, Gkazinou C, Almpanidou V, Hays GC (2017) Global sea turtle conservation successes. Science Adv 3:e1600730. https://doi.org/10.1126/sciadv.1600730
McClenachan L, Jackson JB, Newman MJ (2006) Conservation implications of historic sea turtle nesting beach loss. Front Ecology Environ 4:290–296. https://doi.org/10.1890/1540-9295(2006)4%5B290:CIOHST%5D2.0.CO;2
Meylan PA, Meylan AB, Gray JA (2011) The ecology and migrations of sea turtles 8. Tests of the developmental habitat hypothesis. Bull Am Mus Nat Hist 357:1–70. https://doi.org/10.1206/357.1
Meylan A, Iturbe I, Zurita J, Harrison E, Gray J, Meylan P (2014) Green turtles tagged in benthic developmental habitat in Bermuda nest in Mexico and Costa Rica. Mar Turt Newsl 141:15–17
Meylan A, Hardy RF, Meylan P, Gray J, Shamblin B, Frandsen HR, Shaver DJ (2020) Chelonia mydas (green sea turtle). developmental migration. Herpetol Rev 51:107
Moran KL, Bjorndal KA (2005) Simulated green turtle grazing affects structure and productivity of seagrass pastures. Mar Ecol Prog Ser 305:235–247. https://doi.org/10.3354/meps305235
Mortimer JA (1981) The feeding ecology of the West Caribbean green turtle (Chelonia mydas) in Nicaragua. Biotropica 13:49–58. https://doi.org/10.2307/2387870
Murdoch TJT, Glasspool AF, Outerbridge M, Ward J, Manuel S, Gray J, Nash A, Coates KA, Pitt J, Fourqurean JW, Barnes PA (2007) Large-scale decline in offshore seagrass meadows in Bermuda. Mar Ecol Prog Ser 339:123–130. https://doi.org/10.3354/meps339123
Musick JA, Limpus C (1997) Habitat utilization and migration in juvenile sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, pp 137–163
Nelson WG (2009) Conceptual framework for a review of research needs for development of national water quality criteria protective of seagrasses. Seagrasses and protective criteria: a review and assessment of research status. Office of Research and Development, National Health and Environmental Effects Research Laboratory, EPA/600/R-09/050
Ogden JC, Robinson L, Whitlock K, Daganhardt H, Cebula R (1983) Diel foraging patterns in juvenile green turtles (Chelonia mydas L.) in St. Croix United States Virgin Islands. J Exp Mar Biol Ecol 66:199–205. https://doi.org/10.1016/0022-0981(83)90160-0
Orth RJ, Carruthers TJ, Dennison WC, Duarte CM, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Olyarnik S, Short FT (2006) A global crisis for seagrass ecosystems. Bioscience 56:987–996. https://doi.org/10.1641/0006-3568(2006)56%5B987:AGCFSE%5D2.0.CO;2
Rasheed MA, Unsworth RK (2011) Long-term climate-associated dynamics of a tropical seagrass meadow: implications for the future. Mar Ecol Prog Ser 422:93–103. https://doi.org/10.3354/meps08925
Redfoot W, Ehrhart L (2013) Trends in size class distribution, recaptures, and abundance of juvenile green turtles (Chelonia mydas) utilizing a rock riprap lined embayment at Port Canaveral, Florida, USA, as developmental habitat. Chelon Conserv Biol 12:252–261. https://doi.org/10.2744/CCB-0952.1
Rodriguez AR, Heck KL (2020) Green turtle herbivory and its effects on the warm, temperate seagrass meadows of St. Joseph Bay, Florida (USA). Mar Ecol Prog Ser 639:37–51. https://doi.org/10.3354/meps13285
Sarkis S, Outerbridge ME (2014) Management plan for Bermuda’s resident green and hawksbill sea turtles (Chelonia mydas, Eretmochelys imbricata). Government of Bermuda, Department of Conservation Services, 33pp
Scott AL, York PH, Duncan C, Macreadie PI, Connolly RM, Ellis MT, Jarvis JC, Jinks KI, Marsh H, Rasheed MA (2018) The role of herbivory in structuring tropical seagrass ecosystem service delivery. Front Plant Sci 9:127. https://doi.org/10.3389/fpls.2018.00127
Seminoff JA, Jones TT, Eguchi T, Jones DR, Dutton PH (2006) Stable isotope discrimination between soft tissues of the green sea turtle Chelonia mydas and its diet. Mar Ecol Prog Ser 308:271–278. https://doi.org/10.3354/meps308271
Seminoff JA, Allen CD, Balazs GH, Dutton PH, Eguchi T, Haas H, Hargrove SA, Jensen M, Klemm DL, Lauritsen AM, MacPherson SL (2015) Status review of the green turtle (Chelonia mydas) under the U.S. endangered species Act. NOAA Technical Memorandum, NOAANMFS-SWFSC-539. 571pp
Silva BM, Bugoni L, Almeida BA, Giffoni BB, Alvarenga FS, Brondizio LS, Becker JH (2017) Long-term trends in abundance of green sea turtles (Chelonia mydas) assessed by non-lethal capture rates in a coastal fishery. Ecol Indi 79:254–264. https://doi.org/10.1016/j.ecolind.2017.04.008
Tomasko DA, Dawes CJ (1990) Influences of season and water depth on the clonal biology of the seagrass Thalassia testudinum. Mar Biol 105:345–351
Valentine JF, Heck KL (2021) Herbivory in seagrass meadows: an evolving paradigm. Estuar Coasts 44:491–505. https://doi.org/10.1007/s12237-020-00849-3
Vierros M, Meylan A, Meylan P, Gray J, Ward J (2002) Evaluation of green turtle habitat, population size and distribution using remote sensing and GIS techniques. In: Mosier A, Foley A, Brost B (eds) Proceedings of the twentieth annual symposium on sea turtle biology and conservation. NOAA Technical Memorandum NMFS-SEFSC-477 p. 52–54
Vierros MK (1999) Remote sensing and GIS applications in tropical marine ecosystems. PhD thesis University of North Wales, Bangor
Waycott M, Duarte CM, Carruthers TJ, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci USA 106:12377–12381. https://doi.org/10.1073/pnas.0905620106
Williams SL (1988) Thalassia testudinum productivity and grazing by green turtles in a highly disturbed seagrass bed. Mar Biol 98:447–455. https://doi.org/10.1007/BF00391121
Witherington B, Bresette M, Herren R (2006) Chelonia mydas – green turtle. In: Meylan PA (ed) Biology and conservation of Florida turtles. Chelonian Research Monographs 3, pp 90–104
Witherington B, Hirama S, Hardy R (2012) Young sea turtles of the pelagic Sargassum-dominated drift community: habitat use, population density, and threats. Mar Ecol Prog Ser 463:1–22. https://doi.org/10.3354/meps09970
Zieman JC (1975) Seasonal variation of turtle grass, Thalassia testudinum König, with reference to temperature and salinity effects. Aquat Bot 1:107–1. https://doi.org/10.1016/0304-3770(75)90016-9
Acknowledgements
The Bermuda Turtle Project is a cooperative effort of the Sea Turtle Conservancy (STC, formerly the Caribbean Conservation Corporation), the Bermuda Aquarium Museum and Zoo (BAMZ) and the Bermuda Zoological Society (BZS). For their strong support of the project over its 50-year history, we thank David Godfrey, James Burnett-Herkes, Brian Luckhurst, Wolfgang Sterrer, Patrick Talbot, Ian Walker and Richard Winchell. We thank the captains of RV Chelonia, RV Calamus, and RV Endurance: Campbell O’Connor, John Whiting, Lynch Eastman, Derek Exel, Anson Nash, Tim Hasselbring and Nigel Pollard. For their superb help of many kinds, we thank Debbie Boyer, Beth Brost, Robert Chandler, Chris Flook, Lisa Greene, Lee Ann Hinton, Emma Harrison, Brian Lightbourne, Billy Mitchell, Katherine Nisbett, Barbara Outerbridge, Mark Outerbridge, Lisa Ray, Gäelle Roth, Camilla Stringer, Jack Ward and many other members of the staff of BAMZ. Special thanks to Marjo Vierros for her work on seagrasses during the early years of this study. We were assisted with data collection by numerous volunteers, including over 200 students from 41 different countries who participated in the BTP sea turtle biology and conservation course over 21 years. Numerous community members in Bermuda supported our efforts, especially, the Clee, Harvey, and Wardman families. We thank Karen Bjorndal and Alex Gulick for their comments on an earlier draft and to Jeff Seminoff and an anonymous reviewer for insightful comments on the submitted version.
Funding
The BTP has been funded by the Atlantic Conservation Partnership, the Bermuda Zoological Society, the H. Clay Frick family, the Helen Clay Frick Foundation, and Chevron Bermuda, with additional in-kind support from the Bermuda Aquarium Museum and Zoo, Eckerd College and the Florida Fish and Wildlife Conservation Commission. Open access fees were paid by the Bermuda Zoological Society, Eckerd College and the Sea Turtle Conservancy.
Author information
Authors and Affiliations
Contributions
All authors conceived the study and contributed to the collection of the data set. PAM and RFH conducted the statistical analyses. PAM and ABM led the writing of the manuscript with contributions from JAG and RFH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable guidelines for handling live sea turtles have been followed and all necessary approvals have been obtained. Turtle capture and data collection in Bermuda were authorized under a series of written agreements and permits from the Bermuda Department of the Environment and Natural Resources, most recently, License No. 2018071309. No IACUC agreement was required for this work, however, the same methods are used by PAM and ABM in Panama and have been approved by the Smithsonian Tropical Research IACUC committee, most recently STRI ACUC 2020–0414-2023.
Informed consent
Consent for participation is not applicable to this study as there were no human test subjects.
Additional information
Responsible Editor: P. Casale.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“The point is made that many of the organisms of Bermuda are at or near the extreme poleward limit of their possible tolerance, that they have no margin to spare” (De Laubenfels 1950:158).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meylan, P.A., Hardy, R.F., Gray, J.A. et al. A half-century of demographic changes in a green turtle (Chelonia mydas) foraging aggregation during an era of seagrass decline. Mar Biol 169, 74 (2022). https://doi.org/10.1007/s00227-022-04056-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04056-5