Abstract
Intertidal macrobenthic assemblages associated with monospecific stands of Zostera muelleri, Cymodocea serratula, Halodule uninervis and Halophila ovalis seagrasses are known to display uniform spatial patchiness on the Moreton Bay coast of North Stradbroke Island, Queensland, as do those in Z. capensis in the Knysna estuarine bay, South Africa. Thirty-seven historical datasets of these macrobenthic assemblages were re-analysed to assess variation of local patchiness in each of the 18 most common individual assemblage components at each of these localities in terms of three metrics: overall patchiness (Lloyd's index of patchiness), levels of unoccupancy, and variation in abundance across occupied samples (Lloyd's index of mean crowding). Within-site patchiness was not caused by a restriction of individual species to specific subareas but by variation in their local density, particularly by the extent of unoccupied ‘interstitial’ spaces within patches. Especially in the more uniform Queensland conditions, the more common species occurred relatively widely across the whole locality; individual samples from which a given species was absent never themselves formed patches, the number of such samples conforming to points on truncated normal curves of the frequency of occurrence. Of the 36 species investigated, the two most abundant and widespread both in Queensland and in South Africa displayed significant or near-significant uniformity of levels of local patchiness, whilst five showed significantly uniform mean crowding and ten significantly uniform unoccupancy. This is the first demonstration that some species may display a characteristic level of patchiness in a given habitat type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patchiness is the norm in biological systems; patchiness of the habitat, patchiness of resources, patchiness of organismal numbers (Wiens 1989; Kotliar and Wiens 1990). It also occurs at all spatial scales such that patches are themselves patchy (Kotliar and Wiens 1990; Morrisey et al. 1992). These spatial patterns have been much studied in seagrass systems, particularly in relation to anthropogenically-induced habitat fragmentation (Bell et al. 2001; Rielly-Carroll and Freestone 2017). Some seagrass faunal characteristics do seem resistant to the effects of mosaic habitats, in some areas at least (Lefcheck et al. 2016), but even continuous and apparently uniform beds may support patchy macrofaunal assemblages both in terms of their occupancy and abundance (Kraan et al. 2009; Barnes and Hamylton 2019).
Recently, Barnes (2019a, 2020a) assembled data from a series of > 30 expanses of three species of dwarf-eelgrass from seemingly equivalent intertidal-flat habitats in sheltered bays in the cool-temperate north-west Atlantic, warm-temperate southern Indian Ocean, and subtropical eastern Pacific. The macrobenthic assemblages characterising all these beds of Zostera subgenus Zosterella [= Nanozostera in the 2013 revision of Coyer et al. (2013)] were found to display effectively the same low but significant level of patchiness, a Lloyd's index of 1.125 ± 0.054 (SD). Further, intertidal-flat beds of other seagrass genera (Cymodocea, Halodule and Halophila) were found to support macrofaunal assemblages with levels of patchiness that also fell within one standard deviation of the mean value for those in dwarf-eelgrass (Barnes 2020a). Assemblages from adjacent subtidal meadows and from high-level salt-marsh creeks, however, showed values outside this remarkably uniform range for intertidal beds (Barnes 2019a; Barnes and Claassens 2020).
Such metrics of macrofaunal assemblages are amalgamations of those of all the component species concerned, and these individual elements are known to display a range of dispersions from random to highly patchy at these sites (Barnes and Hamylton 2019). The present study, based on the subtropical eastern Pacific locality and, to a lesser extent, that in the warm-temperate southern Indian Ocean, sought to investigate the null hypothesis that patchiness of each of the more common component species of the benthic macrofauna at these locations, which together create systems with a uniform degree of patchiness, also display a characteristic level of patchiness that remains constant over time and local space. Since notions of patchiness have components of both distribution (presence/absence) and abundance (differential numbers when present) these two components were also investigated in parallel with overall levels of patchiness as determined by Lloyd's index.
Materials and methods
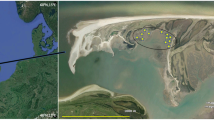
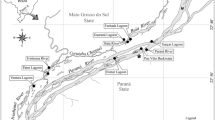
Twenty-seven historical datasets from the period 2009–2019 (see Online Appendix) are available for the seagrass macrobenthos associated with monospecific areas of four seagrass species (Zostera muelleri capricorni, Cymodocea serratula, Halodule uninervis, and Halophila ovalis) along a 6 km stretch of the northwest Moreton Bay coast of North Stradbroke Island (Minjerribah), Queensland (Fig. 1); i.e. from Deanbilla (27° 30′ 32" S, 153° 24′ 33" E), Goompi (27° 29′ 38" S, 153° 23′ 54" E), Yerrol (27° 29′ 02" S, 153° 24′ 19" E), and Capembah (27° 28′ 04" S, 153° 25′ 19" E). These datasets generally comprised series of randomly located samples but those from Deanbilla also included spatially-explicit regular lattices with samples 0.3 m and 5.75 m apart. A further ten historical datasets from the period 2011–2016 (see Online Appendix) are also available for similar sized and to a degree equivalent intertidal extents of a fifth species, Zostera capensis, with macrofaunal samples taken at three points within a distance of 2.5 km across the marine-bay section of the Knysna estuarine system in South Africa’s Western Cape (Fig. 1): Brenton (34° 03′ 35" S, 23° 02′ 07" E), Kingfisher (34° 03′ 39" S, 23° 03′ 09" E), and Steenbok (34° 03′ 47" S, 23° 03′ 25" E). Individual samples from Kingfisher were also spatially explicit, including some with a fixed interval of c. 6 m. Datasets from large (> 1 ha) areas were split into a number of smaller, non-overlapping sets where appropriate to yield a standard series of small local < 2000 m2 sites. Full details of the sampling methodology at each site are given in the papers listed in Online Appendix.
All these macrobenthic seagrass assemblages were located between mean low water and low water spring tide levels, and all were sampled using the same methodology, involving series of 30–80 individual core samples (average 56) per site, each of 0.0054 m2 area and 100 mm depth, together with one further series of 256. Those from Moreton Bay were sampled during the austral spring and those from Knysna in the austral summer. A total of 2279 such samples was analysed. All were from continuous swards of seagrass, were at least 10 m away from any interfaces with adjacent bare sediment, and were collected at low tide before complete tidal ebb whilst the substratum was still covered by at least 15 cm of water. Cores were gently sieved (‘puddled’) through 710 µm mesh on site. This sampling procedure collects the smaller (mostly < 5 mm) and more numerous members of the macrofauna that constitute the large majority of invertebrate biodiversity (Bouchet et al. 2002; Albano et al. 2011), though not the meiofauna nor much scarcer megafauna nor sessile animals attached to the seagrass leaves. Warwick et al. (2006) have shown that different patterning rules may apply to meiofauna and macrofauna, and likewise Davidson et al. (2004) and Leopardas et al. (2014) to sessile species. Sessile or semi-sessile species that had accidentally become detached from the seagrass leaves during sampling were therefore ignored.
Retained material from each core was: (1) placed in a large polythene bag of local seawater within which all seagrass was shaken vigorously to dislodge all but sessile animals; (2) then re-sieved and transported immediately to a local laboratory, and (3) there placed in a 30 × 25 cm tray over a light source in which the living fauna was located by visual examination using 3.5 × magnifying spectacles until no further animal could be observed. Animals were identified to species level wherever possible, with all organismal nomenclature here being as listed in the World Register of Marine Species (www.marinespecies.org) (accessed June 2020). It should be noted, however, that the specific identity of several animals, especially amongst the Polychaeta and Peracarida, is questionable because of lack of relevant systematic studies in Queensland and South Africa. Such animals were treated as morphospecies, an operationally appropriate procedure to detect spatial patterns in numbers of species and their differential abundance (Dethier and Schoch 2006; Gerwing et al. 2020). The 18 most widespread and abundant of the species in both Moreton Bay and Knysna were each subjected to patchiness analyses.
All abundance data for individual species included unoccupied samples (i.e. zero values); occupancies are proportions of the total samples at a given site in which a species was present and, correspondingly, unoccupancies are those from which it was absent. Magnitude of patchiness was ascertained by spatial point pattern analysis of count data using Lloyd's index of patchiness (Lloyd 1967), the ratio of mean crowding (see below) to mean density, given by Ip = [1 + (v − m)/m2], where 'm' is the mean abundance across samples and ‘v’ is the associated spatial variance (Waters et al. 2012; Henriques et al. 2017). A Lloyd's index of 1 indicates a random distribution, whilst one < 1 suggests uniformity and > 1 patchiness. This index has been demonstrated to yield equivalent results to those of the spatially explicit Moran's spatial auto-correlation index for intertidal dwarf-eelgrass macrobenthos (Barnes and Hamylton 2019). It is also independent of sample size over a wide range of areas, provided that the animals position themselves at random with respect to each other within a patch and that the patches are large relative to sample size (Lloyd 1967; Myers 1978). Granted that the core area was 0.0054 m2, it seems unlikely that macrofaunal patches were smaller than that; indeed it has previously been found that cores of 0.0015, 0.0026 and 0.0054 m2 spatial grain all produced the same value of the closely similar but differently-derived Morisita’s Iδ index (Barnes 2016).
It should be noted that Lloyd’s Ip although termed an index of patchiness is actually an index of dispersion; values of such an index that indicate significant departures from random are dependent on the total number of individuals in the dataset, which will vary from species to species and across sites. Hence significant departures from random in the direction of patchiness per se cannot be inferred from any specific value of the index, although all such values will be > 1. Besides the calculation of the Ip index itself, two metrics influencing it were also assessed in parallel: (a) proportion of unoccupied samples (unoccupancy); and (b) variation in abundance where present (i.e. across only occupied samples) using Lloyd's domain-free index of mean crowding (Ic), the mean number of neighbours per individual per unit area, where Ic = m + (v/m − 1) (Lloyd 1967; Wade et al. 2018). Within-site spatial location of unoccupied samples was also assessed in two large Deanbilla datasets, both derived from lattices of 16 × 16 samples, located within (a) 0.85 ha and (b) a nested 23 m2 subarea. Spatial dispersion of unoccupied samples was assessed by nearest-neighbour analysis of spatially explicit data, with the Donnelly (1978) correction for rectangular edge effects (the minimum number of such samples was 47 = 18% of the total). Potentially confusingly, in contrast to Lloyd's IP, nearest-neighbour R > 1 suggests a uniform dispersion, 0 random, and < 1 one that is clustered or patchy.
All calculations were carried out in Microsoft Excel for Mac 16.37 with the StatPlus:mac Pro 7.1.1 add-on, or via PAST 3.24 (Hammer et al. 2019). Correlations were assessed as nonparametric Spearman’s rank (Sr) or parametric Pearson's product-moment coefficient (Pc) as appropriate; and statistical significance of any uniformity of values of patchiness, mean crowding or (after logit transformation) unoccupancy was determined by Monte Carlo simulation with 9999 iterations, yielding a relatively conservative estimate of significant departure from random.
Results
The 18 species from each locality included in this study comprised ≈ 75% of the total seagrass macrobenthic animals sampled. All correlations between the abundances of individual species at each locality were weak (Sr ≤ 0.3), and there was no correlation between Ip value and number of core samples at a site (Moreton Bay: Sr = − 0.09; P > 0.15. Knysna: Sr = 0.05; P > 0.5) or variation in Ip with year of sampling (Moreton Bay: ANOVA F7,231 = 0.88; P > 0.5. Knysna: F5,129 = 1.65; P > 0.15). The Ip values of the known and presumed epifaunal species (largely the gastropod molluscs) were significantly larger than those of the known and presumed infaunal species (principally polychaetes and decapods) (Moreton Bay: ANOVA F1,241 = 21.05, P < 0.00001. Knysna: ANOVA F1,132 = 9.90, P = 0.002). Two genera, the gastropods Alaba and Nassarius, were common to both localities, and average values for the mean and variance of Ip for the Nassarius species were effectively identical: 5.26 and 19.50 for N. burchardii in Moreton Bay and 5.28 and 20.30 for N. kraussianus at Knysna (ANOVA F1,19 = 0.00004, P > 0.995). Those for A. difformis in Moreton Bay and A. pinnae at Knysna were also very similar (3.77 and 5.82 versus 4.09 and 6.85; ANOVA F1,12 = 0.05, P > 0.8) notwithstanding a considerable difference in their abundances (means of > 65 versus < 15 ind m−2).
Of the 18 dominant Moreton Bay species, six were always significantly patchy whilst the other 12 each varied from being randomly distributed to significantly patchy across the different sites. All species occurred across the whole locality through a wide range of spatial scales but with considerable variation in local abundance; the spatial dispersion of a representative species is illustrated in Fig. 2. Their spatial patterns, however, were not in the form of restrictions to species-specific local areas (i.e. geographical ‘patches’). Absences were only from scattered samples (as illustrated in Figs. 2 and 3) and for the more abundant species the number of unoccupancies all conformed to expectation based on truncated normal distributions of frequencies of occurrence (Fig. 4). Using the full (16 × 16 sample) spatially-explicit datasets from Deanbilla, in no species did unoccupied core samples form spatial clusters within sites (i.e. display values of nearest-neighbour R < 1). In all species, individual unoccupied samples were dispersed across the sampled areas of 0.85 ha and 23 m2 either randomly (R not differing significantly from 1; z < 0.6; P > 0.3) or uniformly (R > 1.4; z > 8; P < 0.0001). Uniform dispersions, reflecting the regular pattern of the sampling stations, characterised those, such as Longiflagrum and Eriopisella, with numerous unoccupancies (> 50% of the total samples). The dispersion of unoccupancy in one species, however, depended on the spatial resolution of the analysis. In Eriopisella, decreasing the resolution by four [i.e. from the original 256 samples to 64 (each comprising a block of 4 individual cores, as in Fig. 3)] decreased the value of R at the larger 0.84 ha site from 1.5 (z = 9.1; significantly uniform at P < 0.0001) to 0.87 (z = − 1.4; almost significantly patchy at P = 0.08).
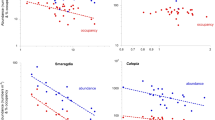
Spatially-explicit diagram of the abundance of a representative member of the Moreton Bay seagrass macrofauna, the benthic amphipod Limnoporeia, across a large (0.85 ha) block of seagrass at Deanbilla (from the data of Barnes and Laurie 2018), and within a small (23 m2) subarea nested within that larger block (data of Barnes and Hamylton 2019). Abundances are displayed as total numbers in blocks of four 0.0054 m2 cores, a notional distance of 11.5 m apart (for 0.85 ha) and 0.6 m (for 23 m2). Overall abundance was 144–200 m−2 (close to average density for the commoner species); Ip at 0.85 ha = 1.37 and at 23 m2 = 1.28, in each case significantly patchy at P < 0.001
Spatially-explicit distribution of unoccupancies (indicated by the white circles) of four representative species at two spatial scales at the Deanbilla site in Moreton Bay [from the data of Barnes and Laurie (2018) and Barnes and Hamylton (2019)]. As in Fig. 2, each datapoint is the total of four 0.0054 m2 core samples
Plots of the frequency of different numbers of individual Enigmaplax per core sample, one of the most numerous species at the Deanbilla site, across an area of 0.85 ha in 2017 and across a nested subarea of 23 m2 in 2018, showing the fitted truncated-normal curves. In each case the number of zero values is no more than would be appropriate in such a distribution granted the mean value [from the data of Barnes and Laurie (2018) and Barnes and Hamylton (2019)]
Table 1 sets out the mean value (together with associated variance) for each of the three patchiness metrics for each of the 18 dominant species across the various Moreton Bay sites. The two most abundant and widespread species across the whole locality, both with a > 70% occupancy and between them with 35% of the 22,200 macrofaunal animals, were the tiny macrophthalmid crab Enigmaplax littoralis and the truncatelloid microgastropod Calopia imitata. Both these, together with the snapping-shrimp Alpheus, showed a uniform level of patchiness across all sites (P < 0.01), Enigmaplax with a mean Ip of 1.19 and Calopia one of 2.07. Eight species, including the Enigmaplax, Calopia and Alpheus above, showed significantly uniform levels of unoccupancy across sites (P < 0.05), and four displayed a similarly uniform degree of mean crowding. Three (Calopia, Limnoporeia and Alpheus) showed uniformity in two of the three measures of patchiness, and one (Enigmaplax) in all three, whilst eight did not display uniformity in any measure. Values of Ip were significantly correlated with those of unoccupancy in half of the 18 species (Sr > 0.40, P < 0.05), and those of Ip with Ic in seven (Sr > 0.45, P < 0.05), Ip being significantly correlated with both unoccupancy and Ic in four (all gastropods). Ip values of two of the more numerous species, the infaunal crustaceans Enigmaplax and Longiflagrum, were correlated with those of Ic (Sr > 0.45, P < 0.05) but not unoccupancy; indeed, the only infaunal species to show a correlation between Ip and unoccupancy was the polychaete Armandia (Sr = 0.74, P < 0.01). Values of Ip across the sites were negatively correlated with a species' abundance at them (P < 0.05) for Armandia (Sr = − 0.75), Smaragdia (Sr = − 0.57), Calopia (Sr = − 0.44) and Alaba (Sr = − 0.83) (and Tricolia approached this state with Sr = − 0.61, P = 0.06), and positively so for Circulus (Sr = 0.73) (P < 0.05). In addition, values of Ip in Dasybranchus were negatively correlated with the total numbers of macrobenthos at the various sites (Sr = − 0.67; P < 0.05). Pearson product-moment correlation revealed particularly strong linear relationships between the Ip values of the dominant microgastropods and of Enigmaplax (P < 0.005): positive between Calopia and Enigmaplax (Pc = 0.55) and between Circulus and Pseudoliotia (Pc = 0.83); negative between Enigmaplax and both Pseudoliotia (Pc = − 0.53) and Circulus (Pc = − 0.87), and between Calopia and Circulus (Pc = − 0.89). An equally strong relationship occurred between the Ip values of Limnoporeia and Dasybranchus (Pc = 0.84).
In respect of the 18 dominant species across the ten Knysna datasets (Table 2), five were always significantly patchy whilst the other 13 each varied from being randomly distributed to significantly patchy dependent on site. There, no species showed a significantly uniform level of patchiness across all sites, although the polychaetes Simplisetia erythraeensis and Prionospio sexoculata (the two most abundant and widespread species at Knysna, together comprising 18% of the total individuals and both with occupancies > 60%) approached this state at P = 0.06–0.07. Those two, together with the annelids Scoletoma tetraura and tubificid sp., and the crustaceans Cyathura estuaria, Danielella edwardsii and ?Cylindroleberis, however, were significantly uniform across sites according to the Smith-Gill (1975) standardised Morisita index (P < 0.05). Only two species, Danielella and gastropod Nassarius, showed significantly uniform levels of unoccupancy across sites (P < 0.05), and only one (the polychaete Orbinia angrapequensis) displayed a similarly uniform degree of mean crowding. Values of Ip and unoccupancy were significantly correlated within four of the 18 species, three epifaunal and one infaunal (Sr > 0.65, P < 0.05), and those of Ic, and Ip were significantly correlated within two of them, both epifaunal (Sr > 0.76, P < 0.05). Values of Ip across the sites were negatively correlated with a species' abundance at them in Exosphaeroma (Sr = − 0.67) and Melita (Sr = − 0.93) (and approached this state in Nassarius with Sr = − 0.61, P = 0.06), and were positively so for Danielella (Sr = 0.79) (P < 0.05). The only particularly strong Pearson correlation (P < 0.005) of between-species Ip values was that between Nassarius and Orbinia (Pc = 0.97).
Discussion
Most investigation of ‘patchiness’ in intertidal marine soft sediments, from Bassindale and Clark (1960) through to Kotta and Möller (2009) and Schenone and Thrush (2020), has reported individual benthic species or associations of species to occur in discrete geographical patches that may overlap with those of other species to varying extents, forming a mosaic or harlequin system in contrast to the classic zonal pattern on rocky shores. The occurrence of benthic species is patchy and their abundance then varies within each such patch. Indeed, several studies have sought to correlate the presence of such specific patches with biological or physical features of the habitat (e.g. Kotta and Möller 2009; Yamada et al. 2014; Jelinski 2015). In the systems under consideration here, at least at the spatial scales investigated, the occurrence of few if any component species seems patchy in that sense. Most species in the Moreton Bay beds are distributed throughout the localities from scales of kilometres down to ones of < 1 m2 (Barnes and Hamylton 2019), and up to 10 of the 12 most numerous faunal elements could occur in a unit 0.0054 m2 sample. Spatially-explicit sampling across a 1 ha area at Deanbilla in Moreton Bay, for example, showed no spatial separation of inhabited and non-inhabited blocks of habitat. Experience elsewhere also suggests that at least amongst Zostera epifauna, patterns experienced at scales of a few metres do not differ from those in areas several kilometres apart (Whippo et al. 2018). This may indicate an important role of seagrass as an agent of biological habitat amelioration in a manner similar to that discussed by Moore et al. (2007) for intertidal algal beds (see, e.g., van Katwijk et al. 2016).
The results also strongly indicate that the degree of patchiness of some individual species, like that of the whole macrobenthic assemblage to which they belong (Barnes and Laurie 2018; Barnes and Hamylton 2019), is effectively constant across local space and overtime periods of a decade. Values of patchiness indices are dependent on quadrat size (Steenweg et al. 2018); those reported here, however, are all of the same grain (0.0054 m2), one appropriate for the size of animals concerned, and cannot have had bearing on the results other than yielding small mean values of density per unit sample with the consequent high probability of chance unoccupancies. The species under study were the relatively numerous ones that characteristically also show correspondingly high levels of occupancy (He and Gaston 2003). Relatively rare species are not a major element of the Knynsa fauna, but they are in Moreton Bay seagrass (Barnes 2014). Not all species with low occupancies display small population densities though, and there is a wide range in abundance of such species. In Moreton Bay, species with very low occupancy (e.g. < 10%) such as the vitrinellid microgastropod Circulus cinguliferus may achieve the highest local density of any member of the macrobenthos, at 37 in a single 0.0054 m2 sample. By virtue of their rare but superabundant hotspots, overall they form one of the most abundant assemblage components. Other species with similarly low occupancies, however, remain at very low density and some have never been recorded in excess of 1 per 0.0054m2 (Barnes 2019b). It may be significant that the two most abundant and most frequently occurring animals, Enigmaplax and Calopia in Moreton Bay and Simplisetia and Prionospio in Knysna, were the ones to show the largest degree of spatial uniformity of dispersion pattern.
Although neither Moreton Bay nor Knysna seagrass appeared a mosaic system, the occurrence of scattered unoccupancies within inhabited blocks was a major influence on the level of macrofaunal patchiness, the greater degree of correlation between Ip and unoccupancy than between Ip and Ic indicating a more important role than that of variation in non-zero density. In a companion study of the interspecific relationship between abundance, occupancy and patchiness within seagrass assemblages (Barnes 2020b), it was also found that whilst Lloyd's Ip and unoccupancy were strongly correlated, Lloyd's Ip and Ic were more poorly so and in several cases the correlation was not statistically significant. Both these inter- and intraspecific results, therefore, highlight the need to differentiate conceptually between those unoccupied samples that represent non-frequented areas between inhabited spatial patches and those that are scattered within inhabited areas, not least those necessarily so because of prevailing population density. An animal like a small amphipod measuring some 8 × 3 mm will occupy an area of some 25 mm2, and a density of such a species of some 200 m−2 will take up only 0.5% of the available space (the mean density of the ten most abundant species across the Moreton Bay locality, excluding the two relatively superabundant Calopia and Enigmaplax, was 250 m−2). On average, a 0.0054 m2 core will capture one individual of such an animal, and hence a considerable number of unoccupancies are to be expected. Clearly, in such circumstances, gross patterns of dispersion are dependent on whether the unoccupied samples are themselves clumped. The same is, of course, true in virtually all animal populations, including at much greater scales amongst the largest and most aggregated of animals: there are spaces between individual elephants in a herd as well as between elephant herds.
Although the Moreton Bay and Knysna sites showed broadly the same pattern, there were differences between them. In particular, the levels of both Lloyd's Ip and unoccupancy were considerably less uniform across the South African estuarine bay. In part, this may be a reflection of marked differences in the macrobenthic assemblages characterising the two intertidal seagrass systems. That in Moreton Bay is a low-density and high species-richness assemblage, whilst that at Knysna supports almost twice the density but less than half the species, and, notwithstanding the general paradigm that seagrass beds are dominated by epifauna (Bouma et al. 2009) as they are indeed in Moreton Bay, the intertidal beds investigated in Knysna were infauna dominated. Nevertheless, such differences do not appear to affect overall levels of macrobenthic assemblage patchiness, which have proved remarkably uniform across all types of intertidal seagrass sward (Barnes 2019a, 2020a). There are however other, and probably more important, differences. The Knysna sample was considerably smaller (10 sites and 602 cores versus 27 sites and 1677 cores), and many of the values from Knysna that closely approached, but did not attain, P < 0.05 are likely to have been significant with larger numbers of individuals and/or sites. The locality itself was also much smaller, more enclosed by land, and more spatially diverse in its local ambient environments. All three sets of Knysna sites were, like those in Moreton Bay, large expanses of marine tidal flat, but, although close together, that at Brenton was relatively open and exposed along the main longitudinal channel, that at Kingfisher was partly sheltered in the mouth of a subsidiary channel, and Steenbok was even more sheltered within a saltmarsh-enclosed channel between an island and the mainland (see Fig. 1). Although this does not impact on overall levels of assemblage patchiness (Barnes 2019a), it might be expected that such local variation in habitat features might differentially affect individual species; it certainly does so with respect to species composition of the assemblages (Barnes and Claassens 2020), and two of the Knysna species are known significantly to change their degree of patchiness along a local environmental gradient (Barnes 2019c).
Causality of the common features that the Moreton Bay and Knysna localities do show can only be speculative. Patterns of low but statistically uniform levels of patchiness across considerable areas and periods of time, however, suggest structuring processes capable of operating over those same scales. Processes operating over such scales must also control the overall very low macrofaunal density in the seagrass (≈ 2,500 ind m−2 in Moreton Bay and 4000 + ind m2 at Knysna), c.f. mean levels of > 40,000 m−2 ecologically-equivalent and similarly-sized animals in areas of the temperate Zostera (Zosterella) noltei seagrass beds of Arcachon Bay in Atlantic France (Blanchet et al. 2004) and > 60,000 m−2 in those of southern North Sea shores (Barnes and Ellwood 2011). Bottom-up process certainly operates in seagrass ecosystems, perhaps particularly those in relatively oligotrophic areas in which seagrass productivity increases in response to nutrient enrichment (York et al. 2018). There is also clear evidence that nektonic predators may be limited by the availability of their macrobenthic prey (Saulnier et al. 2020). Unfortunately there has been little study of the microphytobenthic productivity upon which most of the seagrass macrobenthos feed across a wide range of latitude (Hope et al. 2020), although it seems clear that productivity of the seagrass itself increases towards the tropics (Duarte and Chiscano 1999; Vergés et al. 2018). On the assumption that microphytobenthic productivity follows that of the associated seagrass, the marked mismatch between macrobenthic abundance and the productivity supporting it suggests a much greater importance of top-down control. The results of this study, therefore, could be taken to provide further support to the proposed important predatory role of wide-ranging animals such as the juvenile nekton that use such areas as nurseries (Whitfield 2017, 2020; Lefcheck et al. 2019). Particularly in warmer climates (Freestone et al. 2020), these juvenile nekton opportunistically consume prey items by optimal foraging (Beseres and Feller 2007) and leave, by chance, only the occasional hot-spot of small invertebrate abundance (Beal et al. 2018; Barnes and Hamylton 2019). These types of the area may have served such a nursery role ever since the Devonian (Gess and Whitfield 2020). Few studies, however, have sought to link spatial patterns of potential benthic prey with those of their nektonic (or other) predators (Saulnier et al. 2020), and effectively nothing is known of the population dynamics of the potential prey items in Moreton Bay or Knysna.
In Moreton Bay, dominant crustaceans like Enigmaplax may well be the preferred prey of many of the relatively small predators that roam over the meadows at high tide (see e.g. Morrison et al. 2014), and at Knysna the camptandriids Danielella and Paratylodiplax may fill much the same role there as related macrophthalmid Enigmaplax in Queensland. The dominant gastropod in Moreton Bay, Calopia, although a shelled mollusc and hence not a type of prey item generally regarded as preferred in such habitats (e.g. Reynolds et al. 2018), does have a particularly thin shell and might not be as unpalatable as most other gastropods seem to be (e.g. Vinson and Baker 2008; Sieben et al. 2011). The locally dominant Knysna gastropods have much stronger shells, and that may account for the very great abundance of Alaba pinnae (a mean 30,000 m−2) in subtidal areas there (Barnes and Claassens 2020) despite these being highly accessible to potential nektonic predators. As above, the low overall assemblage densities at Moreton Bay and Knysna generally suggest very heavy mortality of the small animals that dominate the macrobenthos, with mortalities at least equivalent to those documented by Reise (1985), Bachelet and Yacine-Kassab (1987), Beal et al. (2018), etc. in the cooler temperate North Atlantic. Nevertheless, nuanced relationships between the prey items appear to remain: it is not an unselective blanket reduction in density. Thus whilst circumstances leading to maximum patchiness of the two most dominant Moreton Bay species Enigmaplax and Calopia could well be similar, there is a strong negative relationship between Ip patchiness of Enigmaplax and that of the subdominant Pseudoliotia. Of the various microgastropods, and in marked contrast to Calopia, for its size Pseudoliotia has a thick, heavy shell bearing surface sculpture of a type suggested by Vermeij (1974, 1978) to discourage consumption. The same is true of its fellow vitrinellid Circulus. If that is the case, however, relative immunity from predation clearly has not resulted in numerical dominance over the putatively edible Calopia. Polychaetes such as those that dominate the intertidal Knysna seagrasses are little exploitated by fish in the similar nearby Swartvlei estuary (Whitfield 1988), although apogonids, gobiids, sillaginids, sparids and terapontids have all been recorded to feed on the types of worms known to inhabit seagrass beds (Kanou et al. 2005; D’Souza et al. 2009). However, what affects the spatial patterns of distribution and abundance of benthic polychaetes appears poorly understood.
The indications in the results of this study of differential levels of patchiness of epifaunal and infaunal species, and the broadly contrasting patterns of relationship between overall patchiness and unoccupancy between sites and lifestyles, seem particularly worthy of further investigation for the light that they may spread on the causes of patchiness in general. Given the extensive ecosystem services provided by seagrass beds (De los Santos et al. 2020) and their global decline since the 1930s (Connolly et al. 2020), understanding the nature and causes of macrobenthic patchiness within them could be of great importance to their conservation. Granted the small size of the animals concerned, it would also be most instructive to investigate their spatial dispersion at very small spatial intervals, i.e. equivalent to the studies on dispersion of mudflat polychaetes pioneered by Reise (1979).
Data availability
The datasets analysed by the author during the current study are available from the author on reasonable request.
References
Albano PG, Sabelli B, Bouchet P (2011) The challenge of small and rare species in marine biodiversity surveys: microgastropod diversity in a complex tropical coastal environment. Biodivers Conserv 20:3223–3237. https://doi.org/10.1007/s10531-011-0117-x
Bachelet G, Yacine-Kassab M (1987) Intégration de la phase post-recrutée dans la dynamique des populations du gastéropode intertidal Hydrobia ulvae (Pennant). J Exp Mar Biol Ecol 111:37–60. https://doi.org/10.1016/0022-0981(87)90019-0
Barnes RSK (2014) Is spatial uniformity of soft-sediment biodiversity widespread and if so, over what scales? Mar Ecol Progr Ser 504:147–158. https://doi.org/10.3354/MEPS10770
Barnes RSK (2016) Spatial homogeneity of benthic macrofaunal biodiversity across small spatial scales. Mar Environ Res 122:148–157. https://doi.org/10.1016/j.marenvres.2016.10.006
Barnes RSK (2019a) Local patchiness of macrobenthic faunal abundance displays homogeneity across the disparate seagrass systems of an estuarine bay. Mar Environ Res 148:99–107. https://doi.org/10.1016/j.marenvres.2019.05.001
Barnes RSK (2019b) Spatial structure of a multi-species guild: the dominant biofilm-grazing microgastropods of seagrass. Hydrobiologia 827:293–307. https://doi.org/10.1007/s10750-018-3781-y
Barnes RSK (2020b) Interspecific relationship of patchiness to occupancy and abundance, as exemplified by seagrass macrobenthos. Ecol Indic 107083 (in press)
Barnes RSK (2019c) Abundance/occupancy/patchiness relations in estuarine seagrass macrobenthos. Estuar Coast Shelf Sci 228:106360. https://doi.org/10.1016/j.ecss.2019.106360
Barnes RSK (2020) Do different sympatric seagrasses support macrobenthic faunas of differing composition, abundance, biodiversity or patchiness? Mar Environ Res 160:104983. https://doi.org/10.1016/j.marenvres.2020.104983
Barnes RSK, Claassens L (2020) Do beds of subtidal estuarine seagrass constitute a refuge for macrobenthic biodiversity threatened intertidally? Biodivers Conserv 29:3227–3244. https://doi.org/10.1007/s10531-020-02019-0
Barnes RSK, Ellwood MDF (2011) Macrobenthic assemblage structure in a cool-temperate intertidal dwarf-eelgrass bed in comparison to those in lower latitudes. Biol J Linn Soc 104:527–540. https://doi.org/10.1111/j.1095-8312.2011.01738.x
Barnes RSK, Hamylton SM (2019) Isometric scaling of faunal patchiness: seagrass macrobenthic abundance across small spatial scales. Mar Environ Res 146:89–100. https://doi.org/10.1016/j.marenvres.2019.03.011
Barnes RSK, Laurie H (2018) Seagrass macrofaunal abundance shows both multifractality and scale-invariant patchiness. Mar Environ Res 138:84–95. https://doi.org/10.1016/j.marenvres.2018.04.009
Bassindale R, Clarke RB (1960) The Gann Flat, Dale: studies on the ecology of a muddy beach. Field Stud 1(2):1–22. https://fsj.field-studies-council.org/media/350569/vol8.1_214.pdf. Accessed 8 June 2011
Beal BF, Coffin CR, Randall SF, Goodenow CA Jr, Pepperman KE, Ellis BW, Jourdet CB, Protopopescu GC (2018) Spatial variability in recruitment of an infaunal bivalve: experimental effects of predator exclusion on the softshell clam (Mya arenaria L.) along three tidal estuaries in southern Maine, USA. J Shellfish Res 37:1–27. https://doi.org/10.2983/035.037.0101
Bell SS, Brooks RA, Robbins BD, Fonseca M, Hall MO (2001) Faunal response to fragmentation in seagrass habitats: implications for seagrass conservation. Biol Conserv 100:115–123. https://doi.org/10.1016/S0006-3207(00)00212-3
Beseres JJ, Feller RJ (2007) Changes in the spatial distribution of subtidal macrobenthos due to predation by the white shrimp (Litopenaeus setiferus). Estuar Coast 30:591–600. https://doi.org/10.1007/BF02841956
Blanchet H, de Montaudouin X, Lucas A, Chardy P (2004) Heterogeneity of macrozoobenthic assemblages within a Zostera noltii seagrass bed: diversity, abundance, biomass and structuring factors. Estuar Coast Shelf Sci 61:111–123. https://doi.org/10.1016/j.ecss.2004.04.008
Bouchet P, Lozouet P, Maestrati P, Heros V (2002) Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biol J Linn Soc 75:421–436. https://doi.org/10.1046/j.1095-8312.2002.00052.x
Bouma TJ, Ortells V, Ysbaert T (2009) Comparing biodiversity effects among ecosystem engineers of contrasting strength: macrofauna diversity in Zostera noltii and Spartina anglica vegetations. Helgol Mar Res 63:3–18. https://doi.org/10.1007/s10152-008-0133-8
Connolly R, Collier C, O'Leary J, McKenzie L, Ambo-Rappe R, Bandeira S, Kiswara W (2020) Threats to seagrasses and ecosystem resilience. In: United Nations Environment Programme, out of the blue: the value of seagrasses to the environment and to people. UNEP, Nairobi, pp 36–47 (ISBN:978-92-807-3780-6)
Coyer JA, Hoarau G, Kuo J, Tronholm A, Veldsink J, Olsen JL (2013) Phylogeny and temporal divergence of the seagrass family Zosteraceae using one nuclear and three chloroplast loci. Syst Biodivers 11:271–284. https://doi.org/10.1080/14772000.2013.821187
Davidson IC, Crook AC, Barnes DKA (2004) Quantifying spatial patterns of intertidal biodiversity: is movement important? Mar Ecol 25:15–34. https://doi.org/10.1111/j.1439-0485.2004.00015.x
De los Santos CB, Scott A, Arias-Ortiz A, et al. (2020) Seagrass ecosystem services: assessment and scale of benefits. In: United Nations Environment Programme, out of the blue: the value of seagrasses to the environment and to people. UNEP, Nairobi, pp 21–34 (ISBN:978-92-807-3780-6)
Dethier MN, Schoch GC (2006) Taxonomic sufficiency in distinguishing natural spatial patterns on an estuarine shoreline. Mar Ecol Progr Ser 306:41–49. https://doi.org/10.3354/meps306041
Donnelly KP (1978) Simulations to determine the variance and edge effect of total nearest-neighbour distance. In: Hodder IR (ed) Simulation studies in archaeology. Cambridge University Press, Cambridge, pp 91–95 (ISBN 978-0521220255)
D'Souza F, Stoner J, Robb M (eds) (2009) Fish diets and food webs in the Swan-Canning estuary. River science (Western Australian Government Department of Water), issue 28:1.12. https://www.water.wa.gov.au/__data/assets/pdf_file/0010/3106/88049.pdf. Accessed June 2020
Duarte CM, Chiscano CL (1999) Seagrass biomass and production: a reassessment. Aquat Bot 65:159–174. https://doi.org/10.1016/S0304-3770(99)00038-8
Freestone AL, Carroll EW, Papacostas KJ, Ruiz GM, Torchin ME, Sewall BJ (2020) Predation shapes invertebrate diversity in tropical but nor temperate seagrass communities. J Anim Ecol 89:323–333. https://doi.org/10.1111/1365-2656.13133
Gerwing TG, Cox K, Allen Gerwing AM, Campbell L, Macdonald T, Dudas SE, Juanes F (2020) Varying intertidal invertebrate taxonomic resolution does not influence ecological findings. Estuar Coast Shelf Sci 232:106516. https://doi.org/10.1016/j.ecss.2019.106516
Gess RW, Whitfield AK (2020) Estuarine fish and tetrapod evolution: insights from a Late Devonian (Famennian) Gondwanan estuarine lake and a southern African Holocene equivalent. Biol Rev 95:865–888
Hammer Ø, Harper DAT, Ryan PD (2019) PAST: Paleontological statistics software package for education and data analysis, version 3.24. https://folk.uio.no/ohammer/past/past3manual.pdf. Accessed 2 Nov 2019
He F, Gaston KJ (2003) Occupancy, spatial variance and the abundance of species. Am Nat 162:366–375. https://doi.org/10.1086/377190
Henriques DSG, Borges PAV, Gabriel R (2017) Regional processes drive bryophyte diversity and community composition in a small oceanic island. Commun Ecol 18:193–202. https://doi.org/10.1556/168.2017.18.2.9
Hope JA, Paterson DM, Thrush SF (2020) The role of microphytobenthos in soft-sediment ecological networks and their contribution to the delivery of multiple ecological services. J Ecol 108:815–830. https://doi.org/10.1111/1365-2745.13322
Jelinski DE (2015) On a landscape ecology of a harlequin environment: the marine landscape. Landsc Ecol 30:1–6. https://doi.org/10.1007/s10980-014-0109-9
Kanou K, Sano M, Kohno H (2005) Ontogenetic diet shift, feeding rhythm, and daily ration of juvenile yellowfin goby Acanthogobius flavimanus on a tidal mudflat in the Tama River estaury, central Japan. Ichthol Res 52:319–324. https://doi.org/10.1007/s10228-005-0287-1
Kotliar NB, Wiens JA (1990) Multiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity. Oikos 59:253–260. https://doi.org/10.2307/3545542
Kotta J, Möller T (2009) Important scales of distribution patterns of benthic species in the Gretagrund area, the central Gilf of Riga. Est J Ecol 58:259–269. https://doi.org/10.3176/ECO.2009.4.02
Kraan C, van der Meer J, Dekinga A, Piersma T (2009) Patchiness of macrobenthic invertebrates in homogenized intertidal habitats: hidden spatial structure at a landscape scale. Mar Ecol Progr Ser 383:211–224. https://doi.org/10.3354/meps07994
Lefcheck JS, Marion SR, Lombana AV, Orth RJ (2016) Faunal communities are invariant to fragmentation in experimental seagrass landscapes. PLoS ONE 11(5):e0156550. https://doi.org/10.1371/journal.pone.0156550
Lefcheck JS, Hughes BB, Johnson AJ, Pfirrman BW, Rasher DB, Smyth AR, Williams BL, Beck MW, Orth RJ (2019) Are coastal habitats important nurseries? A meta-analysis. Conserv Lett 2019:e12645. https://doi.org/10.1111/conl.12645
Leopardas V, Uy W, Nakaoka M (2014) Benthic macrofaunal assemblages in multispecific seagrass meadows of the southern Philippines: variation among vegetation dominated by different seagrass species. J Exp Mar Biol Ecol 457:71–80. https://doi.org/10.1016/j.jembe.2014.04.006
Lloyd M (1967) Mean crowding. J Anim Ecol 36:1–30. https://doi.org/10.2307/3012
Moore P, Hawkins SJ, Thompson RC (2007) Role of biological habitat amelioration in altering the relative responses of congeneric species to climate change. Mar Ecol Progr Ser 334:11–19. https://doi.org/10.3354/meps334011
Morrisey DJ, Howitt L, Underwood AJ, Stark JS (1992) Spatial variation in soft-sediment benthos. Mar Ecol Progr Ser 81:197–204. https://doi.org/10.3354/meps081197
Morrison MA, Lowe ML, Grant CM, et al. (2014) Seagrass meadows as biodiversity and productivity hotspots. In: Wellington: Ministry for Primary Industries: New Zealand Aquatic Environment and Biodiversity Report No 137 (ISBN 978-0-478-43764-5)
Myers JH (1978) Selecting a measure of dispersion. Environ Entomol 7:619–621. https://doi.org/10.1093/ee/7.5.619
Reise K (1979) Spatial configurations generated by motile polychaetes. Helgoland Wiss Meeres 32:55–72. https://doi.org/10.1007/BF02189892
Reise K (1985) Tidal flat ecology. an experimental approach to species interactions. Springer, Berlin (ISBN 978-3-642-70495-6)
Reynolds PL, Stachowicz JJ, Hovel K et al (2018) Ecology 99:29–35. https://doi.org/10.1002/ecy.2064
Rielly-Carroll E, Freestone AL (2017) Habitat fragmentation differentially affects trophic levels and alters behavior in a multi-trophic marine system. Oecologia 183:899–908. https://doi.org/10.1007/s00442-016-3791-2
Saulnier E, Le Bris H, Tableau A, Dauvin JC, Brind’Amour A (2020) Food limitation of juvenile marine fish in a coastal and estuarine nursery. Est Coast Shelf Sci 241:106670. https://doi.org/10.1016/j.ecss.2020.106670
Schenone S, Thrush SF (2020) Unraveling ecosystem functioning in intertidal soft sediments: the role of density-driven interactions. Sci Rep 10:11909. https://doi.org/10.1038/s41598-020-68869-4
Sieben K, Rippen AD, Eriksson BK (2011) Cascading effects from predator removal depend on resource availability in a benthic food web. Mar Biol 158:391–400. https://doi.org/10.1007/s00227-010-1567-5
Smith-Gill SJ (1975) Cytophysiological basis of disruptive pigmentary patterns in the leopard frog Rana pipiens. II Wild type and mutant cell-specific patterns. J Morphol 146:35–54. https://doi.org/10.1002/jmor.1051460103
Steenweg R, Hebblewhite M, Whittington J, Lukacs P, McKelvey K (2018) Sampling scales define occupancy and underlying occupancy–abundance relationships in animals. Ecology 99:172–183. https://doi.org/10.1002/ecy.2054
van Katwijk MM, Thorhaug A, Marbà N et al (2016) Global analysis of seagrass restoration: the importance of large-scale planting. J Appl Ecol 2016:567–578. https://doi.org/10.1111/1365-2664.12562
Vergés A, Doropoulos C, Czarnik R, McMahon K, Llonch N, Poore AGB (2018) Latitudinal variation in seagrass herbivory: global patterns and explanatory mechanisms. Global Ecol Biogeogr 27:1068–1079. https://doi.org/10.1111/geb.12767
Vermeij G (1974) Marine faunal dominance and mollusc shell form. Evolution 28:656–664. https://doi.org/10.2307/2407289
Vermeij G (1978) Biogeography and adaptation. Patterns of marine life. Harvard University Press, Harvard (ISBN 9780674073760)
Vinson MR, Baker M (2008) Poor growth of rainbow trout fed New Zealand mud snails Potamopyrgus antipodarum. N Am J Fish Manag 28:701–708. https://doi.org/10.1577/M06-039.1
Wade MJ, Fitzpatrick CL, Lively CM (2018) 50 year anniversary of Lloyd’s “mean crowding”: ideas on patchy distributions. J Anim Ecol 87:1221–1226. https://doi.org/10.1111/1365-2656.12854
Warwick RM, Dashfield SL, Somerfield PJ (2006) The integral structure of a benthic infaunal assemblage. J Exp Mar Biol Ecol 330:12–18. https://doi.org/10.1016/j.jembe.2005.12.013
Waters EK, Sidhu HS, Mercer GN (2012) Spatial heterogeneity in simple deterministic SIR models assessed ecologically. Aust N Z Ind Appl Math J 54:23–36. https://doi.org/10.1017/S1446181113000035
Whippo R, Knight NS, Prentice C, Cristiani J, Siegle MR, O’Connor MI (2018) Epifaunal diversity patterns within and among seagrass meadows suggest landscape-scale biodiversity processes. Ecosphere 9(11):e02490. https://doi.org/10.1002/ecs2.2490
Whitfield AK (1988) The fish community of the Swartvlei estuary and the influence of food availability on resource utilization. Estuaries 11:160–170. https://doi.org/10.2307/1351968
Whitfield AK (2017) The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources for fishes in estuaries. Rev Fish Biol Fish 27:75–110. https://doi.org/10.1007/s11160-016-9454-x
Whitfield AK (2020) Littoral habitats as major nursery areas for fish species in estuaries: a reinforcement of the reduced predation paradigm. Mar Ecol Prog Ser 649:219–234. https://doi.org/10.3354/meps13459
Wiens JA (1989) Spatial scaling in ecology. Funct Ecol 3:385–397. https://doi.org/10.2307/2389612
Yamada K, Tanaka Y, Era T, Nakaoka M (2014) Environmental and spatial controls of macroinvertebrate functional assemblages in seagrass ecosystems along the Pacific coast of northern Japan. Glob Ecol Conserv 2:47–61. https://doi.org/10.1016/j.gecco.2014.08.003
York PH, Hyndes GA, Bishop MJ, Barnes RSK (2018) Faunal assemblages of seagrass ecosystems. In: Larkum AWD, Kendrick GA, Ralph PJ (eds) Seagrasses of Australia. Structure ecology and conservation. Springer, Cham, pp 541–588. https://doi.org/10.1007/978-3-319-71354-0_17
Acknowledgements
I reiterate my gratitude to the Queensland Parks and Wildlife Service, the Quandamooka Yoolooburrabee Aboriginal Corporation and the Quandamooka Aboriginal Land and Sea Management Agency, and South Africa National Parks Scientific Services and Knysna Office, for permission to sample under permit in the Moreton Bay Marine Park and Quandamooka People's native title area, Australia, and in the Garden Route National Park, South Africa; and to the Moreton Bay Research Station and Knysna Basin Project Field Laboratory for hospitality and the use of facilities.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflicts of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study were followed and all necessary permissions and approvals were obtained in respect of the original collections of the data.
Additional information
Responsible Editor: F. Bulleri.
Reviewed by G.M. Martins and an undisclosed expert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barnes, R.S.K. Do species display characteristic intraspecific levels of patchiness in a given habitat type? The case of intertidal seagrass macrobenthos. Mar Biol 167, 177 (2020). https://doi.org/10.1007/s00227-020-03793-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03793-9