Abstract
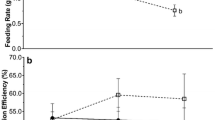
The influence of dietary elemental contents on consumer stoichiometry was investigated in selected and combined soft tissues (as a proxy of the whole individual) of the omnivorous sea urchin, Lytechinus variegatus. We raised urchins for 4 months in controlled seawater tanks using three different diets with different nutritional contents (from lower to higher: seagrass, red macroalgae, and a formulated diet). Individuals fed the different diets varied an average of 19.7, 19.4, and 38 % in C:N, C:P, and N:P ratios, respectively, with stronger temporal variability for C:P and N:P ratios across tissues and whole individuals. This resulted in homeostasis parameters (1/H) of −0.45, 0.09, and 0.38, respectively, for C:N, C:P, and N:P, indicative of homeostatic to weakly homeostatic organisms, at least for C:P and N:P ratios. Individuals fed the nutrient-rich formulated diet had higher growth rates (14 ± 0.83 g WW month−1) than those fed macroalgae or seagrass (9.3 ± 0.57 and 3.4 ± 0.33 g WW month−1, respectively). However, rapid body increments in more nutritional diets caused both a decrease in the %N and an increase in the %P of soft tissues, which resulted in significant but opposite effects of diet stoichiometry and growth in sea urchin C:N (R = −0.74 and R = 0.93, for diet and growth effects, respectively) and N:P ratios (R = 0.60 and R = −0.63, also, respectively, for diet and growth effects). Among potential compensatory mechanisms helping to preserve certain levels of homeostasis, ingestion rates (g WW diet per g WW of urchin) were higher for seagrass and macroalgae diets than for the nutrient-rich formulated diet. In contrast, absorption and growth efficiencies displayed significant negative associations with nutrient contents in diets and did not exhibit nutritional compensation. Overall, our results suggest that resource stoichiometry strongly determines the growth rate of individuals (R = 0.88, P < 0.01), and moderate variability in C:N:P ratios of sea urchins possibly arise from differences in the allocation of proteins and RNA to body components, similarly to what has been proposed by the growth rate hypothesis.






Similar content being viewed by others
References
Baggett LP, Heck KL Jr, Frankovich TA, Armitage AR, Fourqurean JW (2013) Stoichiometry, growth, and fecundity responses to nutrient enrichment by invertebrate grazers in sub-tropical turtle grass (Thalassia testudinum) meadows. Mar Biol 160:169–180. doi:10.1007/s00227-012-2075-6
Bauer G, Schulze ED, Mund M (1997) Nutrient contents and concentrations in relation to growth of Picea abies and Fagus sylvatica along a European transect. Tree Physiol 17:777–786. doi:10.1093/treephys/17.12.777
Beddingfield SD, McClintock JB (1998) Differential survivorship, reproduction, growth and nutrient allocation in the regular echinoid Lytechinus variegatus (Lamarck) fed natural diets. J Exp Mar Biol Ecol 226:195–215
Boersma M, Elser JJ (2006) Too much of a good thing: on stoichiometrically balanced diets and maximal growth. Ecology 87(5):1325–1330. doi:10.1890/0012-9658(2006)87[1325:TMOAGT]2.0.CO;2
Boersma M, Schöps C, McCauley E (2001) Nutritional quality of seston for the freshwater herbivore Daphnia galeata x hyalina: biochemical versus mineral limitations. Oecologia 129:342–348. doi:10.1007/s004420100728
Cazcarra RF, Petit M (2010) The influence of animal age and sward height on the herbage intake and grazing behavior of Charolais cattle. Anim Sci 61(3):497–506. doi:10.1017/S1357729800014065
Cebrian J, Lartigue J (2004) Patterns of herbivory and decomposition in aquatic and terrestrial ecosystems. Ecol Monogr 74:237–259. doi:10.1890/03-4019
Cebrian J, Shurin JB, Borer ET, Cardinale BJ, Ngai JT, Smith MD, Fagan WF (2009) Producer Nutritional Quality Controls Ecosystem Trophic Structure. PLoS One 4:e4929. doi:10.1371/journal.pone.0004929
Chrzanowski TH, Grover JP (2008) Element content of Pseudomonas fluorescens varies with growth rate and temperature. Limnol Oceanog 53:1242–1251
Cronin G, Paul VJ, Hay ME, Fenical W (1997) Are tropical herbivores more resistant than temperate herbivores to seaweed chemical defenses? Diterpenoid metabolites from Dictyota acutiloba as feeding deterrents for tropical versus temperate fishes and sea urchins. J Chem Ecol 23:289–302. doi:10.1023/B:JOEC.0000006360.36833.13
Cruz-Rivera E, Hay ME (2000) Can quantity replace quality? Food choice, compensatory feeding, and fitness of marine mesograzers. Ecology 81:201–219. doi:10.1890/0012-9658(2000)081[0201:CQRQFC]2.0.CO;2
Darchambeau F, Faerovi PJ, Hessen DO (2003) How Daphnia copes with excess carbon in its food. Oecologia 136:336–346. doi:10.1007/s00442-003-1283-7
DeMott WR, Gulati RD, Siewertsen K (1998) Effects of phosphorus-deficient diets on the carbon and phosphorus balance of Daphnia magna. Limnol Oceanogr 43:1147–1161
DePriest MS, Lin SM, Lopez-Bautista JM (2011) Phycological Society of America Annual Meeting. Seattle, WA, USA
Elser JJ, Dobberfuhl DR, MacKay NA, Schampel JH (1996) Organism size, life history, and N: P stoichiometry. Bioscience 46(9):674–684. doi:10.2307/1312897
Elser JJ, Fagan WF, Denno RF, Dobberfuhl DR, Folarin A, Huberty A, Interlandi S, Kilham SS, McCauleyk E, Schulz KL, Siemann EH, Sterner RW (2000) Nutritional constraints in terrestrial and freshwater food webs. Nature 408:578–580. doi:10.1038/35046058
Elser JJ, Acharya K, Kyle M, Cotner J, Makino W, Markow T, Watts T, Hobbie S, Fagan W, Schade J, Hood J, Sterner RW (2003) Growth rate–stoichiometry couplings in diverse biota. Ecol Lett 6:936–943. doi:10.1046/j.1461-0248.2003.00518.x
Færøvig PJ, Hessen DO (2003) Allocation strategies in crustacean stoichiometry: the potential role of phosphorus in the limitation of reproduction. Freshw Biol 48(10):1782–1792. doi:10.1046/j.1365-2427.2003.01128.x
Fernandez C, Boudouresque CF (2000) Nutrition of the sea urchin Paracentrotus lividus (Echinodermata: Echinoidea) fed different artificial food. Mar Ecol Prog Ser 204:131–141. doi:10.3354/meps204131
Fink P, Von Elert E (2006) Physiological responses to stoichiometric constraints: nutrient limitation and compensatory feeding in a freshwater snail. Oikos 115:484–494. doi:10.1111/j.2006.0030-1299.14951.x
Frost PC, Xenopoulos MA, Larson JH (2004) The stoichiometry of dissolved organic carbon, nitrogen and phosphorus release by a planktonic grazer, Daphnia. Limnol Oceanog 49:1802–1808. doi:10.4319/lo.2004.49.5.1802
Frost PC, Evans-White MA, Finkel Z, Jensen TC, Matzek V (2005) Are you what you eat? Physiological constraints on organismal stoichiometry in an elementally imbalanced world. Oikos 109:18–28. doi:10.1111/j.0030-1299.2005.14049.x
Goecker ME, Heck KL Jr, Valentine JF (2005) Effects of nitrogen concentrations in turtlegrass Thalassia testudinum on consumption by the bucktooth parrotfish Sparisoma radians. Mar Ecol Prog Ser 286:239–248. doi:10.3354/meps286239
Hammer HS (2006) Determination of dietary protein, carbohydrate, and lipid requirements for the sea urchin Lytechinus variegatus fed semi-purified feeds. PhD dissertation. University of Alabama at Birmingham, Alabama, USA
Hammer HS, Powell ML, Jones WT, Gibbs VK, Lawrence AL, Lawrence JM, Watts SA (2012) Effect of feed protein and carbohydrate levels on feed intake, growth, and gonad production of the sea urchin Lytechinus variegatus. J World Aqua Soc 43(2):145–158. doi:10.1111/j.1749-7345.2012.00562.x
Heck KL Jr, Valentine JF (2007) The primacy of top-down effects in shallow benthic ecosystems. Estuar Coasts 30:371–381. doi:10.1007/BF02819384
Heflin LE, Gibbs VK, Powell ML, Makowsky R, Lawrence AL (1816) Lawrence JM (2012) Effect of diet quality on nutrient allocation to the test and Aristotle’s lantern in the sea urchin Lytechinus variegatus (Lamarck. J Shell Res 31(3):867–874. doi:10.2983/035.031.0335
Hessen DO (1990) Carbon, nitrogen and phosphorus status in Daphnia at varying food conditions. J Plank Res 12:1239–1249. doi:10.1093/plankt/12.6.1239
Hood II JM (2010) Consumer nutrient stoichiometry: patterns, homeostasis, and links with fitness. PhD dissertation, University of Minnesota
Klumpp DW, Nichols PD (1983) Nutrition of the southern sea garfish Hyporhamphus melanochir: gut passage rate and daily consumption of two food types and assimilation of seagrass components. Mar Ecol Prog Ser 12:207–212
Larson BR, Vadas RL, Keser M (1980) Feeding and nutritional ecology of the sea urchin Strongylocentrotus drobachiensis in Maine, USA. Mar Biol 59:49–62
Laspoumaderes C, Modenutti B, Balseiro E (2010) Herbivory versus omnivory: linking homeostasis and elemental imbalance in copepod development. J Plank Res 0:1–10. doi:10.1093/plankt/fbq077
Lawrence JM, Klinger TS (2001) Digestion in sea urchins. In: Lawrence JM (ed) Edible sea urchins: biology and ecology. Elsevier Scientific BV, The Netherlands, pp 103–113
Lebrato M, Iglesias-Rodriguez D, Feely RA, Greeley D, Jones DOB, Suarez-Bosche N, Lampitt RS, Cartes JE, Green DRH, Alker B (2010) Global contribution of echinoderms to the marine carbon cycle: CaCO3 budget and benthic compartments. Ecol Monogr 80:441–467. doi:10.1890/09-0553.1
Logan JD, Joern A, Wolesensky W (2004) Control of CNP homeostasis in herbivore consumers through differential assimilation. Bull Math Biol 66:707–725. doi:10.1016/j.bulm.2003.10.008
Lowe EF, Lawrence JM (1976) Absorption efficiencies of Lytechinus variegatus (Lamarck) (Echinodermata: Echinoidea) for selected marine plants. J Exp Mar Biol Ecol 21:223–234
Malzahn AM, Boersma M (2012) Effects of poor food quality on copepod growth are dose dependent and non-reversible. Oikos 121:1408–1416. doi:10.1111/j.1600-0706.2011.20186.x
Malzahn AM, Aberle N, Clemmesen C, Boersma M (2007) Nutrient limitation of primary producers affects planktivorous fish condition. Limnol Oceanogr 52:2062–2071. doi:10.4319/lo.2007.52.5.2062
Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Ann Rev Ecol Syst 11:119–161
Meunier CL, Hantzsche FM, Cunha-Dupont AÖ, Haafke J, Oppermann B, Malzahn A, Boersma M (2012) Intraspecific selectivity, compensatory feeding and flexible homeostasis in the phagotrophic flagellate Oxyrrhis marina: three ways to handle food quality fluctuations. Hydrobiologia 680(1):53–62
Moore H, Jurate T, Bauer J, Jones J (1963) The biology of Lytechinus variegatus (Lamarck). Bull Mar Sci Gulf Carib 13:23–53
Moss JE, Lawrence JM (1972) Changes in carbohydrate, lipid, and protein levels with age and season in the sand dollar Mellita quinquiesperforata (Z ske). J Exp Mar Biol Ecol 8:225–239
Peduzzi P (1987) Dietary preferences and carbon absorption by two grazing gastropods, Gibbula urnbillcaris (Linne) and Jujubinus striatus (Linne) PSZNI. Mar Ecol 8:359–370
Persson J, Fink P, Goto A, Hood JM, Jonas J, Kato S (2010) To be or not to be what you eat: regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 119:741–751. doi:10.1111/j.1600-0706.2009.18545.x
Prado P, Heck KL Jr (2011) Seagrass selection by omnivorous and herbivorous consumers: determining factors. Mar Ecol Prog Ser 429:45–55. doi:10.3354/meps09076
Prado P, Tomas F, Alcoverro T, Romero J (2007) Extensive direct measurements of Posidonia oceanica defoliation confirm the importance of herbivory in temperate seagrass meadows. Mar Ecol Prog Ser 340:63–71. doi:10.3354/meps340063
Prado P, Alcoverro T, Romero J (2010) Influence of nutrients in the feeding ecology of seagrass (Posidonia oceanica L.) consumers: a stable isotopes approach. Mar Biol 157:715–724. doi:10.1007/s00227-009-1355-2
Prado P, Carmichael RH, Watts SA, Cebrian J, Heck KL Jr (2012) Diet-dependent δ13C and δ15N fractionation among sea urchin Lytechinus variegatus tissues: implications for food web models. Mar Ecol Prog Ser 462:175–190. doi:10.3354/meps09786
Rueda AA, Slansky F Jr, Wheeler GS (1991) Compensatory feeding response of the slug Sarasinula plebeia to dietary dilution. Oecologia 88:181–188. doi:10.1007/BF00320809
Sokal RR, Rohlf FJ (1995) Biometry: the principals and practice of statistics in biological research. Freeman, W.H., and Company, New York
Stachowicz JJ, Hay M (1999) Reduced mobility is associated with compensatory feeding and increased diet breadth of marine crabs. Mar Ecol Prog Ser 188:169–178. doi:10.3354/meps188169
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press
Tessier AJ, Goulden CE (1982) Estimating food limitation in cladoceran populations. Limnol Oceanog 27:707–717
Urabe J, Watanabe Y (1992) Possibility of N or P limitation for plaktonic cladocerans: an experimental test. Limnol Oceanogr 37(2):244–251
Valentine JF, Heck KL Jr (2001) The role of leaf nitrogen content in determining turtlegrass (Thalassia testudinum) grazing by a generalized herbivore in the northeastern Gulf of Mexico. J Exp Mar Biol Ecol 258:65–86. doi:10.1016/S0022-0981(00)00342-7
Velimirov B (1984) Grazing of Sarpa salpa (L.) on Posidonia oceanica and utilization of soluble compounds. In: Boudouresque CF, de Grissac J, Olivier J (eds) International workshop in Posidonia oceanica beds, vol 1. GIS Posidonie Publications, France, pp 381–387
Villar-Argaiz M, Medina-Sánchez JM, Carrillo P (2002) Linking life history strategies and ontogeny in crustacean zooplankton: implications for homeostasis. Ecology 83(7):1899–1914. doi:10.1890/0012-9658(2002)083[1899:LLHSAO]2.0.CO;2
Watts SA, McClintock JB, Lawrence JM (2007) The ecology of Lytechinus variegatus. In: Lawrence JM (ed) Edible sea urchins: biology and ecology. Elsevier Press, New York, pp 473–498
Zanotto FP, Simpson SJ, Raubenheimer D (1993) The regulation of growth by locust through post-ingestive compensation for variation in the levels of dietary protein and carbohydrate. Physiol Entomol 18:425–434. doi:10.1111/j.1365-3032.1993.tb00617.x
Acknowledgements
P.P. was supported by a postdoctoral scholarship from the Ramón Areces Foundation, and partial support of this work was provided by NOAA MARFIN and NOAA Northern Gulf Institute grants to K.L.H. and a NOAA NCDDC grant to J.C. We are grateful to Dr. Ruth Carmichael for technical support with the acid wash technique, and to Professor Robert W. Sterner for advice on the interpretation of negative homeostasis coefficients. We thank the Tech Support team at the Dauphin Island Sea Lab for helping us maintain the appropriate physicochemical conditions in experimental tanks, and the staff of the Carmichael and Watts laboratories for supplying advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Rights and permissions
About this article
Cite this article
Prado, P., Heck, K.L. & Cebrian, J. Moderate stoichiometric homeostasis in the sea urchin Lytechinus variegatus: effects of diet and growth on C:N:P ratios. Mar Biol 161, 2869–2883 (2014). https://doi.org/10.1007/s00227-014-2552-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2552-1