Abstract
The aim of this study was to determine the changes occurring in the wood cellulose of the fast-growing poplar (Populus deltoides × maximowiczii) under the influence of steam explosion (SE) pretreatment. Cellulose from native wood and after pretreatment at 160 and 205 °C was isolated. Cellulose polymerization degree by size exclusion chromatography (SEC) and cellulose crystallinity index by Fourier transform infrared spectroscopy-attenuated total reflectance (FTIR-ATR) were determined. The profiles of sugars in the native wood and in the solid fraction after pretreatment (using the acid hydrolysis method) were also determined. In addition, the profile of monosaccharides in the liquid fraction obtained after steam explosion and in the liquid fraction after acid hydrolysis of the oligosaccharides were investigated using high-performance liquid chromatography (HPLC). This allowed to determine the change in the yield of hexoses and pentoses in the studied material.
The behavior of cellulose in wood subjected to steam explosion at 160 and 205 °C and isolated by the Kürschner–Hoffer method was studied by determining the absorption bands of FTIR-ATR spectra. The lateral order index (LOI) of cellulose was calculated from the ratio of the intensity of the corresponding absorption bands A1422/A896 cm−1. Total crystallinity index (TCI) of cellulose was calculated from the ratio of the intensity of absorption bands A1372/A2900 cm−1. TCI of Kürschner-Hoffer cellulose isolated from wood subjected to steam explosion at 160 and 205 °C decreased by 5.6 and 5.0%, respectively, with regard to the applied temperature. LOI increased in cellulose isolated from wood subjected to steam explosion at 160 °C (by 0.7%) and at 205 °C (by 19.2%) in relation to the index of cellulose isolated from native wood. Kürschner–Hoffer cellulose isolated from wood subjected to steam explosion at 160 and 205 °C exhibited, respectively, a reduced degree of polymerization of about 11% and about 8%. Polydispersity index in Kürschner–Hoffer cellulose was 1% lower after both pretreatments than native sample.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The wood biomass is a lignocellulosic complex consisting of glucose-based polysaccharide chain polymers—cellulose, pentose- and hexose-based polymers—hemicelluloses and aromatic polymer-like molecules—lignin (Ralph et al. 2008; Hendriks and Zeeman 2009). Both polysaccharides and lignin are the building materials of cell walls, and besides them there are extractives with different functions and separable using appropriate solvents.
The cellulose molecule is composed of β-d-glucopyranose mers linked by β-1,4-glucosidic bonds (Fengel and Wegener 2003; Klemm et al. 2005). The di-anhydro-d-glucopyranose form a mer repeating n-times in the cellulose molecule, accounting for its degree of polymerization. In the formula of the cellulose molecule, the two extreme glucosidic rings differ from the others, at the fourth carbon atom the –OH group is characterized by reducing properties, and at the first carbon it does not have these properties.
In wood, the degree of polymerization of cellulose is even about 15,000, and its average degree of polymerization varies from 9000 to 10,000 (Goring and Timell 1962; Fengel and Wegener 2003).
Under the influence of physico-chemical and mechanical factors acting on wood, the degree of polymerization of cellulose is significantly reduced. The action of high temperatures on cellulose causes a decrease in its degree of polymerization (Radomski et al. 2010; Antczak et al. 2016; Kučerová et al. 2016a, b; Zawadzki et al. 2016).
If cellulose molecules are arranged parallel to each other at small distances, then they are connected to each other by intermolecular hydrogen bonds. Hydrogen bonds are a form of association between a highly electronegative atom and a hydrogen atom bound by a covalent bond to an electronegative atom. In the cellulose molecule, the electronegative atom is an oxygen atom.
Currently, cellulose is assigned a crystalline-amorphous structure, containing crystalline and amorphous regions. Two cellulose molecules pass through a series of crystalline (oriented) areas, where the cellulose molecules are arranged parallel to each other, and areas of less oriented molecules (amorphous areas). In addition to crystalline and amorphous areas, paracrystalline areas characterized by an intermediate structure between crystalline and amorphous areas may be present in cellulose (Atalla 1993).
Several crystallographic variations of cellulose are known and are numbered with Roman numerals. Cellulose I is found in natural fibers and in this structure the cellulose molecules are arranged in parallel layers. The molecules in each layer are held together by hydrogen bonds, and the cohesion of adjacent layers is maintained by van der Waals forces. Cellulose II is the most thermodynamically stable type of cellulose and is mainly obtained from cellulose I, by mercerization (alkali treatment and regeneration, solubilization and subsequent recrystallization). Cellulose III and Cellulose IV can be formed from cellulose I and II, respectively, by treatment with liquid ammonia and reaction is reversible (Hayashi et al. 1975). Cellulose V and cellulose VI can be obtained by heating cellulose III and IV, respectively (Gardiner and Sarko 1985).
Glucose is a simple sugar formed mainly by the hydrolysis of cellulose, although a small amount is associated with the presence of heterogeneous hemicelluloses.
Cellulose, due to its crystalline-amorphous structure, is characterized by the difficult property of being stiff and elastic at the same time. High degree of crystallinity negatively influences its hydrolysis process. In contrast, amorphous cellulose exhibits from 3 to 30 times the efficiency of this process with respect to crystalline cellulose (Lynd et al. 2002). The crystalline areas of cellulose are resistant to chemicals and to the process of hydrolysis, and this occurs in the amorphous areas (Zhu et al. 2008). They are also temperature resistant, with the process in amorphous areas occurring above 150 °C and in crystalline areas above 180 °C (Yu et al. 2010). In the available literature there is a scarce information about using FTIR-ATR method to crystallinity analysis of cellulose from fast-growing poplars wood in a context of bioethanol production. Hence, in this respect the use of the above method to study the crystallinity can be interesting and noteworthy.
In wood, the polysaccharides accompanying cellulose are hemicelluloses, the molecules of which can be built from monomers of homogeneous and heterogeneous simple sugars. The most common monomers in hemicelluloses molecules are pentoses: β-d-xylopyranose, α-d-arabinofuranose and hexoses: β-d-mannopyranose, β-d-glucopyranose, β-d-galactopyranose (Kačík and Solár 2000; Radomski et al. 2010). In this group of polysaccharides can be distinguished pentosans and hexosans and polyuronides. Among the pentosans, the main polysaccharide is xylan, whose molecule is built from β-d-xylopyranose residues, linked by an oxygen bridge 1,4. On average, seven out of ten d-xylopyranose units have acetyl groups in the C-2 or C-3 position, and every tenth unit is attached by a substituent 4-O-methylglucuronic α-1,2 bond (Fengel and Wegener 2003).
Hemicelluloses show less resistance to physico-chemical agents compared to cellulose. They dissolve in alkaline media and hydrolyze more readily when exposed to dilute acids, and they degrade at range from 160 to 220 °C (Boonstra et al. 2007; Antczak et al. 2019, 2022).
Steam explosion is a physical and chemical method for pretreatment of lignocellulosic biomass (Brownell et al. 1986; Balan et al. 2020; Mankar et al. 2021). This method combines mechanical treatment with chemical treatment caused by wood autohydrolysis. The autohydrolysis of wood involves acetyl groups present in hemicelluloses and the water environment, which under conditions of elevated temperature and pressure have an action similar to that of acids. Autohydrolysis at elevated temperature results in reduction of hemicelluloses and plasticization of lignin, which forms a complex with hemicelluloses. This results in exposing the cellulose structure and reducing its degree of polymerization (Alvira et al. 2010; Tomás-Pejó et al. 2014; Kučerová et al. 2020). The polymerization degree of cellulose is a key parameter influencing the enzymatic hydrolysis of lignocellulosic biomass (Karimi and Taherzadeh 2016). It is known, that the shorter cellulose chains are more reactive to the enzymes, because the shorter chains contain lower hydrogen bonds and are more accessible to enzymes. Additionally, the reduction of cellulose polymerization degree by a pretreatment process causes a formation of more cellulose ends available to the exoglucanase. Based on literature information (Yang et al. 2011) no clear relation has been observed between polymerization degree of cellulose and enzymatic hydrolysis yield. Hence, the research undertaken in this area is still valid and worth attention (Chandra et al. 2007).
The aim of this study was the investigation of glucose and xylose yields from Populus deltoides × maximowiczii wood before and after steam explosion pretreatment. Additionally, the polymerization degree of cellulose, polydispersity and total crystallinity index (TCI) and lateral order index (LOI) were studied.
Materials and methods
Materials
Wood of Populus deltoides × maximowiczii was obtained from 5-year-old stems cut at the end of the growing season and grown on plantations of the Department of Plant Genetics, Breeding and Biotechnology, Faculty of Horticulture, Biotechnology and Landscape Architecture, Warsaw University of Life Sciences, located in Wolica (52°08′42''N, 21°04′07''E). The wood was debarked and then chipped on a laboratory cutting mill. Tests were conducted on wood shavings with a fraction of the 0.43–1.02 mm fraction. They were dried to a moisture content of 5%, and before being pretreated with a steam explosion they were moistened to remove air from the wood pores. The weighed wood shavings were placed in a beaker and flooded with distilled water to 220 cm3 by placing the beaker on a magnetic stirrer with a heating plate, heating to 90 °C for 20 min. Pretreatment by steam explosion was carried out in a high-pressure autoclave with a capacity of 250 cm3, made of acid-resistant steel (Antczak et al. 2022; Gałązka and Szadkowski 2021). Pretreatment with a steam explosion was carried out at 160 and 205 °C, without maintaining the temperature (once the target temperature was reached, there was a quick decompression of the system). Three repetitions were performed for every process temperature. The native material, the solid fraction obtained after steam explosion treatment at 160 and 205 °C, and the liquid fraction formed after pretreatment at both temperatures were used for chemical determinations. The pretreatment was carried out at the Department of Wood Science and Wood Protection, Warsaw University of Life Sciences in Poland. Chemical determinations were made at the Technical University in Zvolen, Slovakia. For this purpose, samples of the solid fraction were packed tightly into plastic containers and the resulting liquid fraction was frozen. The tightly packed material was transported between units and prepared there for further determinations.
Methods
Acid hydrolysis of native wood and solid fraction obtained after pretreatment
In order to determine the yield of monosaccharides (glucose and xylose as the most promising for the production of biofuels from lignocellulosic materials), acid hydrolysis of wood before pretreatment and the solid fraction formed after pretreatment by steam explosion at 160 and 205 °C was carried out. Wood samples before and after steam explosion pretreatment were dried to constant weight at 104 ± 1 °C. Then, 0.1 g of the sample was placed in a 50 cm3 flask and flooded with 1 cm3 of 72% sulfuric acid (VI) (H2SO4) (Chempur, Gliwice, Poland). The samples were heated for 1 h at 30 °C. After adding 28 cm3 of distilled water, they were heated for 2 h at 120 °C in an oil bath (glycerin, Agroekolab, Zvolen, Slovakia), and then cooled to room temperature. The hydrolysates were quantitatively poured into 50 cm3 volumetric flasks and made up to the mark with distilled water. From the solutions obtained, 5 cm3 of each were taken and transferred to beaker and neutralized with barium carbonate (BaCO3) to neutral pH, added 1 cm3 of 5% cellobiose solution (an internal standard), it was filtered and washed with distilled water. The prepared samples were evaporated on a vacuum evaporator (Laborota 4000, Schwabach, Germany). Then the precipitate obtained was dissolved in 1 cm3 of ultrapure water and filtered through a nylon filter with a pore diameter of 0.25 µm and subjected to HPLC (high-performance liquid chromatography) analysis (Kačík and Solár 2000; Kučerová and Výbohová, 2018; Kučerová et al. 2020). Each of the chemical determinations was carried out with triplicate, and standard deviations were determined.
Direct analysis of liquid fraction obtained after pretreatment
The determination of free monosaccharides (glucose and xylose) in the liquid fraction obtained after steam explosion at 160 and 205 °C was carried out by taking 5 cm3 of the solution, which was transferred to a conical flask, adding 1 cm3 of 5% cellobiose solution as an internal standard. After neutralization with BaCO3 to neutral pH, the solution was evaporated on a vacuum evaporator (Laborota 4000, Schwabach, Germany) to obtain a precipitate. The precipitate was dissolved in 1 cm3 of ultrapure water and filtered through a 0.25 µm pore diameter nylon filter and analyzed by HPLC (Kačík and Solár 2000; Kučerová and Výbohová, 2018; Kučerová et al. 2020). Each of the chemical determinations was carried out with triplicate, and standard deviations were determined.
Acid hydrolysis of liquid fraction obtained after pretreatment
In order to analyze the total yield of glucose and xylose in the liquid fraction obtained after pretreatment of Populus deltoides × maximowiczii wood subjected to steam explosion at 160 and 205 °C, assuming the presence of oligosaccharides in this liquid, their hydrolysis was performed. For this purpose, 20 cm3 of solution was taken by placing in 50 cm3 spherical flasks. 0.42 cm3 of 96% sulfuric acid (VI) was added to the solution and placed under a reflux condenser above the hob for 4 h (the liquid boils). Then the solution was quantitatively transferred into 25 cm3 volumetric flasks and made up to the mark with distilled water. From the solutions obtained, 5 cm3 of each was taken and transferred to a beaker and neutralized with BaCO3 to neutral pH, 1 cm3 of 5% cellobiose solution (an internal standard) was added, then filtered and washed with distilled water. The prepared samples were evaporated on a vacuum evaporator (Laborota 4000, Schwabach, Germany). Then the precipitate obtained was dissolved in 1 cm3 of ultrapure water and filtered through a nylon filter with a pore diameter of 0.25 µm and subjected to HPLC analysis (Kučerová and Výbohová, 2018; Kučerová et al. 2020). Each of the chemical determinations was carried out in triplicate, and standard deviations were determined.
HPLC analysis of monosaccharides
Determination of glucose and xylose yield was carried out with an Agilent 1200 HPLC (Agilent Technologies, CA, USA) with a Benson BP-800 Pb column (Benson Polymeric Inc., Reno, NV, USA) at 80 °C, flow rate of 0.6 cm3/min and using refractive index detector. The mobile phase was ultrapure water. All analysis was performed four times per sample, and single standard deviations were calculated (Kačik and Solár 2000; Antczak et al. 2022).
SEC analysis of cellulose isolated from native wood and solid fraction obtained after pretreatment
The degree of cellulose polymerization was measured by size exclusion chromatography (SEC) method. Cellulose samples were isolated using the Kürschner–Hoffer method (three cycles of a mixture of ethanol and nitric acid (V) were used to obtain cellulose) (Saeman et al. 1954; Krutul 2002; Gałązka and Szadkowski 2021) from native wood and solid fraction obtained after SE pretreatment. Before cellulose isolation, the samples were previously extracted with a chloroform-ethanol (93:7)w/w mixture (Antczak et al. 2006). Molecular weight distribution analysis of the cellulose samples was performed after their conversion into cellulose tricarbanilates (Kučerová et al. 2016a, b; Čabalová et al. 2009; Kačík et al. 2009). Cellulose tricarbanilates were dissolved in tetrahydrofuran and filtered through a Puradisc 25 NYL filter (Whatman International) with a pore size of 0.45 µm. SEC analysis using a DAD detector was performed at 35 °C with tetrahydrofuran at a flow rate of 1 cm3/min on a two connected PLgel, 10 μm, 7.5 × 300 mm, MIXED-B columns (Agilent Technologies) preceded by a PLgel, 10 μm, 7.5 × 50 mm, Guard-column (Agilent Technologies). Data acquisitions were carried out with ChemStation software (Agilent Technologies) and calculations were performed with the Clarity GPC module (Data Apex). Numerical outputs were obtained for Mn (the number average molar mass), Mw (the weight average molar mass) and the values were recalculated to underivatized cellulose using Eqs. (1, 2).
where \(k = \frac{{162\;{\text{g}}\;{\text{mol}}^{ - 1} }}{{519\;{\text{g}}\;{\text{mol}}^{ - 1} }}\)
Degree of polymerization (DPw) values were calculated by dividing the weight average molar mass of cellulose by the monomer equivalent mass of anhydroglucose using Eq. (3).
Polydispersity index (PDI) of cellulose was calculated as the ratio of the weight average molar mass to the number average molar mass using Eq. (4).
Measurements were performed four times per sample, and single standard deviations were calculated.
FTIR-ATR analysis
Fourier transform infrared spectroscopy-attenuated total reflectance (FTIR-ATR) spectra of native wood, solid fractions obtained after SE pretreatment and cellulose isolated from these materials were recorded on Nicolet iS10 FTIR spectrometer equipped with Smart iTR attenuated total reflectance (ATR) sampling accessory with diamond crystal (Thermo Fisher Scientific, Madison, WI, USA). Spectra were measured in the wavenumber range from 4000 to 650 cm−1. A resolution of 4 cm−1 and 32 scans per sample were used. Measurements were performed on six replicates per sample and average spectra were created and evaluated. The obtained spectra of the wood samples were normalized at 1031 cm−1. The OMNIC 8.0 software (Thermo Fisher Scientific, Madison, WI, USA) was used to evaluate the spectra (Kačík and Solár 2000; Výbohová et al. 2018).
Results and discussion
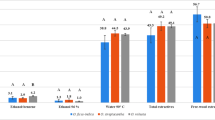
The glucose and xylose yield obtained after acid hydrolysis of native wood and SE pretreated solid fractions is presented in Fig. 1a. On the basis of the data presented in Fig. 1a, it can be concluded that the steam explosion process of Populus deltoides × maximowiczii wood, compared to native wood, increased the glucose yield from 434.2 to 455.5 mg/g (160 °C) and 501.8 mg/g (205 °C), respectively. However, the xylose yield decreased after pretreatment from 135.7 to 130.7 mg/g (160 °C) and 110.0 mg/g (205 °C).
Glucose is a simple sugar formed mainly by the hydrolysis of cellulose, although a small amount of glucose content is related to its presence in heterogeneous hemicelluloses, i.e., glucomannan. During the pretreatment process of Populus deltoides × maximowiczii wood, some saccharides pass into the post-reaction liquid. Figure 1b shows the data of glucose and xylose yield in the liquid fraction obtained after SE of wood at 160 and 205 °C. From the data shown in Fig. 1b, it can be seen that the glucose yield in the post-reaction liquid obtained at 205 °C was over three times higher with respect to its yield in the post-reaction liquid obtained at 160 °C. In contrast, the xylose yield in the post-reaction liquid obtained at 205 °C was about eighteen times higher with respect to its yield in the post-reaction liquid obtained at 160 °C.
The total glucose and xylose yield was obtained by concentrating the post-reaction liquid and then hydrolyzing it with 72% H2SO4. The data presented in Fig. 1c show that the total yield of glucose in the liquid fraction obtained after wood pretreatment did not differ significantly from each other and was 31.6 mg/g (160 °C) and 29.3 mg/g (205 °C), respectively. On the other hand, the total xylose yield increased almost seven times when wood was pretreated at 205 °C with respect to its total yield (16.0 mg/g) in the liquid fraction obtained at 160 °C. The data obtained are consistent with the results of Carvalheiro et al. (2008).
The data of Marchwicka et al. (2015) showed that the glucose content of 3-year-old Populus deltoides × maximowiczii subjected to enzymatic hydrolysis at 24, 72, 150, 216 and 360 h without pretreatment increased gradually from 11.1 to 14.6%. On the other hand, if the wood was pretreated followed by enzymatic hydrolysis using the above-mentioned times, the glucose content also increased gradually and was from 15.6 to 24.1%. The increase in glucose content in wood subjected to steam explosion followed by enzymatic hydrolysis ranged from 28 to 40% compared to the control wood. From the data of Antczak et al. (2014), the glucose content of the wood of Populus deltoides × maximowiczii after extraction in chloroform-ethanol (93:7)w/w mixture and acid hydrolysis was 40.9%. From the above data, it can be concluded that the glucose yield from wood depends on the conditions and processes to which it is subjected.
The xylose content after treatment of Populus deltoides × maximowiczii wood is related to its xylan content, although it also occurs as a component of e.g., glucuronoxylan. Hemicelluloses under steam explosion treatment at 205 °C are degraded and transformed to liquid and gaseous products.
Analyzing the results presented in Fig. 1b, c it can be seen that the SE pretreatment produces oligosaccharides which are not detectable under the applied conditions by HPLC analysis without additional acid hydrolysis. The total glucose yield after acid hydrolysis in the liquid fraction obtained at 160 °C was almost eight times higher and at 205 °C about twice higher than the yield of this sugar in the liquid fraction without additional acid hydrolysis. On the other hand, the total yield of xylose in the post-reaction liquid obtained at 160 °C after acid hydrolysis was over fourteen times higher and at 205 °C was over five times higher than the yield of this sugar in the liquid fraction without acid hydrolysis. Comparing the results shown in Fig. 1a, b, c, it can be observed that hemicelluloses are less heat resistant than cellulose (Rowell 2005; Gawron et al. 2014; Antczak et al. 2022).
One of the methods allowing tracing the changes occurring in the wood of Populus deltoides × maximowiczii before and after steam explosion at 160 and 205 °C is the analysis of FTIR-ATR (Figs. 2 and 3). The interpretation of FTIR spectra was performed based on the literature (Hon and Shiraishi 2001; Kubovský et al. 2020; Bhagia et al. 2022). Celluloses isolated by the Kürschner–Hoffer method from native wood and from solid fraction obtained after steam explosion at 160 and 205 °C were used for the analysis. This method was chosen because it degrades cellulose less than the Seifert method and isolated cellulose is purer than cellulose obtained by Cross-Bevan method (Kačík and Solár 2000; Krutul 2002).
During the thermal treatment of wood, several processes take place simultaneously with different effects on the intensity of the absorption band with a maximum in the wavenumber at about 1736 cm−1. All components of the wood contribute to the change in the intensity of this band, but in most cases, lignin and hemicelluloses are the most important.
Based on the data of changes in the intensity of the absorbance band at the wavenumber 1736 cm−1 it can be concluded that in the wood subjected to steam explosion, there was a decrease in the intensity of the absorbance band by 3.6% (160 °C) and 60% (205 °C) (Fig. 3) compared to the wood not subjected to this process, which indicates that at 205 °C xylan degrades more and goes into solution. On the other hand, the data of Výbohová et al. (2018) show that during the process of the heat treatment of ash wood there was an increase in the absorbance of this peak.
For aromatic skeletal vibrations plus C=O stretch at 1593 cm−1, and aromatic skeletal C=C vibrations at 1507 cm−1 the same decrease was detected for both process temperatures. At the temperature of 160 °C it was 7% for both absorption bands, and at 205 °C approximately 30%. The action on ash wood temperature 160, 180 and 200 °C increased the intensity of the peak 1593 cm−1, which may be due to the relative increase of lignin content in treated wood (Chen et al. 2012; Výbohová et al. 2018).
From the above data, it is evident that in Populus deltoides × maximowiczii wood during high temperature pretreatment, lignin plasticizes and condenses with cellulose and hemicelluloses according to Fengel and Wegener (2003), Hill (2006), Nuopponen et al. (2004), Sun et al. (2016) and Antczak et al. (2018).
The decrease in the intensity of the absorption band at 1462 cm−1, for aromatic C–H deformation and asymmetric bending of groups –CH3 and –CH2- saccharides at process temperatures of 160 and 205 °C was about 60 and 20% respectively, with respect to native wood, indicating transformation of lignin and polysaccharides.
At the wavenumber of 1235 cm−1 for syringyl ring and C–O stretching in lignin and xylan the reduction in intensity of this band in relation to native wood, respectively, to the applied temperature of 160 and 205 °C in the wood of Populus deltoides × maximowiczii subjected to the steam explosion was 7 and 53% (Fig. 3).
Based on the above data, it can be concluded that xylan was degraded and passed into solution after pretreatment of wood at 205 °C, while structural changes occurred in lignin. With increasing temperature, degradation and hydrolysis as well as dissolution of hemicelluloses occur, most of which goes into solution, and at the same time some of them are transformed into gaseous products (Jӧnsson and Martín 2016; Brethauer et al. 2020; Antczak et al. 2022).
Changes occurring in the cellulose isolated from Populus deltoides × maximowiczii wood by Kürschner–Hoffer method, subjected to a steam explosion at 160 and 205 °C, were investigated by analyzing the absorption bands of FTIR-ATR spectra. The cellulose crystallinity index was calculated from absorption bands TCI A1372/A2900 cm−1 and LOI A1423/A896 cm−1 (Fig. 4a, b). Band 2900 cm−1 corresponds to stretching vibrations of –CH in –CH2– and –CH3 groups, while 1372 cm−1 to deformation vibration of C–H in cellulose (Poletto et al. 2012; Geffert et al. 2017). An empirical crystallinity index also known as lateral order index (LOI) was defined as ratio between intensity of the bands at 1422 cm−1 (C–H bending vibrations) and at 897 cm−1 (β-(1–4)-glycosidic bond and C–H deformation vibrations in cellulose) (Nelson and O'Connor 1964; Kumar et al. 2014).
The TCI is proportional to the degree of crystallinity of cellulose in wood and the LOI is correlated to the overall degree of order in the cellulose (O’Connor et al. 1958; Carrillo et al. 2004; Kumar et al. 2014).
Figure 4a shows data as a LOI A1422−1/A896 cm−1 of Kürschner–Hoffer cellulose, from which it can be concluded that cellulose isolated from Populus deltoides × maximowiczii wood subjected to steam explosion at 205 °C was characterized by a 16.1% increase in LOI with respect to the cellulose index from native wood. On the other hand, the cellulose isolated from wood subjected to steam explosion at 160 °C characterized by a 0.6% increase in LOI with respect to the cellulose isolated from native wood. Based on the results, it can be concluded that the steam explosion pretreatment at 205 °C of Populus deltoides × maximowiczii wood affected the increase of crystalline regions in cellulose isolated by the Kürschner–Hoffer method.
According to Auxenfans et al. (2017), a native poplar wood exhibited an LOI of 1.77 ± 0.4 and decreased by about 18% after steam explosion of the biomass. From the data presented in Fig. 4a, b, it can be concluded that the changes in cellulose crystallinity index TCI and LOI in Populus deltoides × maximowiczii wood subjected to steam explosion depended on the applied temperature of the biomass pretreatment process.
On the basis of the data presented in Fig. 4b, it can be concluded that the total crystalline index of cellulose isolated from wood by the Kürschner–Hoffer method, decreased with a comparable rate in the case of both treatment temperatures, namely approximately by 5%. Geffert et al. (2017) reported that the total crystalline index investigated in steamed beech wood increased by 13.2% compared to native wood. This is probably due to the partial degradation of hemicelluloses and amorphous cellulose regions by the influence of high temperature.
Yildiz and Gümüşkaya (2007) found that cellulose isolated from spruce and beech wood samples treated by thermal modification at 150, 180 and 200 °C by 6 and 10 h was characterized by an increase of TCI and LOI. As a result of these modifications, it was concluded that hemicelluloses and less ordered cellulose deteriorated and as a consequence the degree of cellulose crystallinity increased.
According to the literature and the analyzed data, the impact of a steam explosion on wood at 160 and 205 °C resulted in the degradation of hemicelluloses, cellulose with a lower degree of polymerization, and the degradation and change in the structure of lignin. As a result, the observed change in the total crystallinity index was low.
One of the important characteristics of cellulose is its degree of polymerization. The cellulose with degree of polymerization above 200 (i.e., α-cellulose) is characterized by higher resistance to acids, alkalis in comparison to β-cellulose (degree of polymerization around 200) and γ-cellulose (degree of polymerization below 100). Figure 5a shows the polydispersity index data of Kürschner–Hoffer cellulose isolated from Populus deltoides × maximowiczii wood before and after steam explosion at 160 and 205 °C. Figure 5b shows the degree of polymerization of cellulose after steam explosion at 160 and 205 °C.
Figure 5b shows an increase in the share of cellulose with a higher degree of polymerization as the pretreatment temperature increases.
Based on the data presented, it can be concluded that after taking into account the standard deviation, the cellulose isolated from pretreated wood had a similar polydispersity index with respect to the cellulose isolated from native wood. The data obtained are consistent with the results relating to slight changes in the degree of polymerization of cellulose (Alvira et al. 2010; Tomás-Pejó et al. 2011; Sun et al. 2016).
In summary, it can be stated that for cellulose isolated from wood before and after pretreatment by steam explosion at 160 and 205 °C by the Kürschner–Hoffer method, a slight decrease in TCI was observed for cellulose isolated from wood after pretreatment, while for LOI it increased with increasing treatment temperature. In the case of polydispersity index and degree of cellulose polymerization, a decrease in these parameters was observed for pretreated cellulose relative to the native material. The cellulose isolated from wood after pretreatment at 205 °C had higher polymerization and polydispersity index than the cellulose obtained from wood after treatment at 160 °C.
Conclusion
Based on the data presented, it can be concluded that:
-
1.
The change in cellulose structure was observed as a result of the pretreatment by steam explosion at 160 and 205 °C applied to Populus deltoides × maximowiczii wood.
-
2.
Pentosans (xylan) were not resistant to the temperature of 160 and 205 °C used in the steam explosion of wood, hence the xylose yield in the liquid fraction was three times higher than the glucose yield.
-
3.
TCI (A1372/A1900 cm−1) of Kürschner–Hoffer cellulose isolated from Populus deltoides × maximowiczii wood subjected to steam explosion at 160 and 205 °C decreased by 5.6 and 5%, respectively, to the applied temperature relative to the crystallinity index of Kürschner–Hoffer cellulose isolated from native wood.
-
4.
LOI (A1429/A896 cm−1) of Kürschner–Hoffer cellulose isolated from Populus deltoides × maximowiczii wood subjected to steam explosion at 205 °C increased by 19% with respect to the index of cellulose isolated from native wood, whereas Kürschner–Hoffer cellulose isolated from wood subjected to steam explosion at 160 °C did not differ highly in index (LOI) from cellulose isolated from native wood.
-
5.
Kürschner–Hoffer cellulose isolated from Populus deltoides × maximowiczii wood subjected to steam explosion at 160 and 205 °C had a reduced degree of polymerization of about 11% and about 8%, respectively, according to the temperature used.
-
6.
Kürschner–Hoffer cellulose isolated from Populus deltoides × maximowiczii wood subjected to steam explosion at 160 and 205 °C had a similar polydispersity index with respect to cellulose isolated from native wood.
-
7.
The higher temperature of the steam explosion process results in the decomposition of saccharides with lower degrees of polymerization and crystallinity thus allowing for higher values of these parameters than the process at lowers.
References
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861. https://doi.org/10.1016/j.biortech.2009.11.093
Antczak A, Radomski A, Zawadzki J (2006) Benzene substitution in wood analysis. Ann Warsaw Agric Univ Wood Technol 58:15–19
Antczak A, Spyszewska N, Michałuszko A, Kłosińska T, Archanowicz E (2014) Acid hydrolysis of poplar wood (Populus sp.). Przem Chem 93:1428–1431. https://doi.org/10.12916/przemchem.2014.1428
Antczak A, Radomski A, Drożdżek M, Zawadzki J, Zielenkiewicz T (2016) Thermal ageing of cellulose with natural and synthetic antioxidants under various conditions. Drewno 59:139–152. https://doi.org/10.12841/wood.1644-3985.134.10
Antczak A, Marchwicka M, Szadkowski J, Drożdżek M, Gawron J, Radomski A, Zawadzki J (2018) Sugars yield obtained after acid and enzymatic hydrolysis of fast-growing poplar wood species. BioResources 13:8629–8645. https://doi.org/10.15376/biores.13.4.8629-8645
Antczak A, Świerkosz R, Szeniawski M, Marchwicka M, Akus-Szylberg F, Przybysz P, Zawadzki J (2019) The comparison of acid and enzymatic hydrolysis of pulp obtained from poplar wood (Populus sp.) by the Kraft method. Drewno 62:53–66. https://doi.org/10.12841/wood.1644-3985.D07.01
Antczak A, Szadkowski J, Szadkowska D, Zawadzki J (2022) Assessment of the effectiveness of liquid hot water and steam explosion pretreatments of fast-growing poplar (Populus trichocarpa) wood. Wood Sci Technol 56:87–109. https://doi.org/10.1007/s00226-021-01350-1
Atalla RH (1993) The Structures of Native Celluloses. Foundation for Biotechnical and Industrial Fermentation Research. Espoo, Finland
Auxenfans T, Crônier D, Chabbert B, Paës G (2017) Understanding the structural and chemical changes of plant biomass following steam explosion pretreatment. Biotechnol Biofuels 10:36. https://doi.org/10.1186/s13068-017-0718-z
Balan R, Antczak A, Brethauer S, Zielenkiewicz T, Studer MH (2020) Steam explosion pretreatment of beechwood. Part 1: comparison of the enzymatic hydrolysis of washed solids and whole pretreatment slurry at different solid loadings. Energies 13:3653. https://doi.org/10.3390/en13143653
Bhagia S, Ďurkovič J, Lagaňa R, Kardošová M, Kačík F, Cernescu A, Schäfer P, Yoo ChG, Ragauskas AJ (2022) Nanoscale FTIR and mechanical mapping of plant cell walls for understanding biomass deconstruction. ACS Sustain Chem Eng 10:3016–3026. https://doi.org/10.1021/acssuschemeng.1c08163
Boonstra JM, Van Acker J, Tjeerdsma BF, Kegel EV (2007) Strength properties of thermally modified softwoods and its relation to polymeric structural wood constituents. Ann Sci 64:679–690. https://doi.org/10.1051/forest:2007048
Brethauer S, Antczak A, Balan R, Zielenkiewicz T, Studer MH (2020) Steam explosion pretreatment of beechwood. Part 2: quantification of cellulase inhibitors and their effect on Avicel hydrolysis. Energies 13:3638. https://doi.org/10.3390/en13143638
Brownell HH, Yu EKC, Saddler JN (1986) Steam explosion pretreatment of wood: effect of chip size, acid, moisture content and pressure drop. Biotechnol Bioeng 28:792–801. https://doi.org/10.1002/bit.260280604
Čabalová I, Kačík F, Sivák J (2009) Changes of molecular weight distribution of cellulose during pulp recycling. Acta Fac Xyl 51:11–17
Carrillo F, Colom X, Suñol JJ, Saurina J (2004) Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur Polym J 40:2229–2234. https://doi.org/10.1016/j.eurpolymj.2004.05.003
Carvalheiro F, Duarte L, Gírio F (2008) Hemicellulose biorefineries: a review on biomass pretreatments. J Sci Ind Res 67:849–864
Chandra R, Bura R, Mabee W, Berlin A, Pan X, Saddler J (2007) Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics? Adv Biochem Eng Biotechnol 108:67–69. https://doi.org/10.1007/10_2007_064
Chen Y, Gao J, Fan Y, Tshabalala MA, Stark NM (2012) Heat-induced chemical and color changes of extractive-free black locust (Robinia pseudoacacia) wood. BioResources 7:2236–2248. https://doi.org/10.15376/biores.7.2.2236-2248
Fengel D, Wegener G (2003) Wood. Chemistry, ultrastructure, reactions. VK, Remagen
Gałązka A, Szadkowski J (2021) Enzymatic hydrolysis of fast-growing poplar wood after pretreatment by steam explosion. Cell Chem Technol 55:637–647. https://doi.org/10.35812/CelluloseChemTechnol.2021.55.52
Gardiner ES, Sarko A (1985) Packing analysis of carbohydrates and polysaccharides. The crystal structures of celluloses IVI and IVII. Can J Chem 63:173–180. https://doi.org/10.1139/v85-027
Gawron J, Antczak A, Borysiak S, Zawadzki J, Kupczyk A (2014) The study of glucose and xylose content by acid hydrolysis of ash wood (Fraxinus excelsior L.) after thermal modification in nitrogen by HPLC method. BioResources 9:3197–3210. https://doi.org/10.15376/biores.9.2.3197-3210
Geffert A, Výbohová E, Geffertová J (2017) Characterization of the changes of colour and some wood components on the surface of steamed beech wood. Acta Fac Xyl 59:49–57. https://doi.org/10.17423/afx.2017.59.1.05
Goring DAI, Timell TE (1962) Molecular Weight of Native Celluloses. Tappi 5:454–460
Hayashi J, Sufoka A, Ohkita J, Watanabe S (1975) The confirmation of existences of cellulose IIII, IIIII, IVI, and IVII by the X-ray method. J Polym Sci Polym Lett Ed 13:23–27. https://doi.org/10.1002/pol.1975.130130104
Hendriks AT, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresource Technol 1000:10–18. https://doi.org/10.1016/j.biortech.2008.05.027
Hill CAS (2006) Wood modification: chemical, thermal and other processes, John Wiley & Sons Ltd, Chichester
Hon DNS, Shiraishi N (2001) Wood and cellulosic chemistry. Marcel Dekker, New York
Jӧnsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effect. Bioresour Technol 199:103–112. https://doi.org/10.1016/j.biortech.2015.10.009
Kačík F, Solár R (2000) Analytical chemistry of wood. Technical University in Zvolen, Zvolen
Kačík F, Kačíková D, Jablonský M, Katuščák S (2009) Cellulose degradation in newsprint paper ageing. Polym Degrad Stab 94:1509–1514. https://doi.org/10.1016/j.polymdegradstab.2009.04.033
Karimi K, Taherzadeh MJ (2016) A critical review of analytical methods in pretreatment of lignocelluloses: composition, imaging, and crystallinity. Bioresour Technol 200:1008–1018. https://doi.org/10.1016/j.biortech.2015.11.022
Klemm D, Heublein B, Fink H, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393. https://doi.org/10.1002/anie.200460587
Krutul D (2002) Exercises in wood chemistry and selected issues in organic chemistry. SGGW, Warsaw
Kučerová V, Výbohová E (2018) Release of saccharides during hot-water pretreatment of willow wood (Salix alba L.). Cell Chem Technol 52:381–386
Kubovský I, Kačíková D, Kačík F (2020) Structural changes of oak wood main components caused by thermal modification. Polymers 12:485. https://doi.org/10.3390/polym12020485
Kučerová V, Lagaňa R, Výbohová E, Hýrošová T (2016) The effect of chemical changes during heat treatment on the color and mechanical properties of fir wood. BioResources 11:9079–9094. https://doi.org/10.15376/biores.11.4.9079-9094
Kučerová V, Výbohová E, Čaňová I, Ďurkovič J (2016b) The effects of both insoluble lignin and the macromolecular traits of cellulose on the content of saccharides within solids during hydrothermal pretreatment of hybrid poplar wood. Ind Crops Prod 91:22–31. https://doi.org/10.1016/j.indcrop.2016.06.021
Kučerová V, Výbohová E, Honig V, Čabalová I (2020) Chemical changes within solids during liquid hot water pretreatment of wood. BioResources 15:38–48. https://doi.org/10.15376/biores.15.1.38.48
Kumar A, Negi YS, Choudhary V, Bhardwaj NK (2014) Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J Mater Phys Chem 2:1–8. https://doi.org/10.12691/jmpc-2-1-1
Lynd LR, Weimer PJ, van Zyl WHV, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. https://doi.org/10.1128/MMBR.66.3.506-577.2002
Mankar AR, Pandey A, Modak A, Pant KK (2021) Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour Technol 334:125235. https://doi.org/10.1016/j.biortech.2021.125235
Marchwicka MM, Radomski A, Antczak A, Szadkowski J, Lewandowska A, Szadkowska D, Zielenkiewicz T, Drożdżek M, Archanowicz EI (2015) Effect of poplar (Populus sp.) biomass pretreatment on the yield of its enzymatic hydrolysis. Przem Chem 9:814–817. https://doi.org/10.15199/62.2015.5.34
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J Appl Polym Sci 8:1325–1341. https://doi.org/10.1002/app.1964.070080323
Nuopponen M, Willför S, Jääskeläinen A-S, Sundberg A, Vuorinen T (2004) A UV resonance Raman (UVRR) spectroscopic study on the extractable compounds of Scots pine (Pinus sylvestris) wood: Part I: Lipophilic compounds. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 60(13):2953–2961. https://doi.org/10.1016/j.saa.2004.02.008
O’Connor RT, DuPré EF, Mitcham D (1958) Applications of infrared absorption spectroscopy to investigations of cotton and modified cottons: Part I: physical and crystalline modifications and oxidation. Text Res J 28:382–392. https://doi.org/10.1177/004051755802800503
Poletto M, Zattera AJ, Santana RMC (2012) Structural differences between wood species: evidence from chemical composition, FTIR spectroscopy, and thermogravimetric analysis. J Appl Polym Sci 126:E337–E344. https://doi.org/10.1002/app.36991
Radomski A, Zawadzki J, Drożdżek M, Szadkowski J (2010) Non-degradation nitration of pinewood cellulose. Ann Warsaw Agric Univ Wood Technol 72:213–219
Ralph J, Brunow G, Harris P, Dixon RA, Schatz PF, Boerjan W (2008) Lignification: Are lignins biosynthesized via simple combinatorial chemistry or via proteinaceous control and template replication? In: Lattanzio V, Daayf F, Lattanzio V (eds) Recent Advances in Polyphenol Research. Blackwell Publishing Ltd, London, pp 36–66. https://doi.org/10.1002/9781444302400.ch2
Rowell RM (2005) Handbook of Wood Chemistry and Wood Composites. CRC Press, Boca Raton. https://doi.org/10.1201/9780203492437
Saeman JF, Moore WE, Mitchell RL, Millet MA (1954) Techniques for the determination of pulp constituents by quantitative paper chromatography. TAPPI 37:336–343
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58. https://doi.org/10.1016/j.biortech.2015.08.061
Tomás-Pejó E, Alvira P, Ballesteros M, Negro MJ (2011) Pretreatment technologies for lignocellulose to bioethanol conversion. In: Pandey A, Larroche Ch, Ricke SC, Dussap C-G, Gnansounou E (eds) Biofuels. Academic Press, Amsterdam, pp 149–176. https://doi.org/10.1016/B978-0-12-385099-7.00007-3
Tomás-Pejó E, Bonander N, Olsson L (2014) Industrial yeasts strains for biorefinery solutions: Constructing and selecting efficient barcoded xylose fermenting strains for ethanol. Biofuels Bioprod Bioref 8:626–634. https://doi.org/10.1002/bbb.1472
Výbohová E, Kučerová V, Andor T, Balážová Ž, Veľková V (2018) The effect of heat treatment on the chemical composition of ash wood. Bioresources 13:8394–8408. https://doi.org/10.15379/biores.13.4.8394-8408
Yang B, Dai Z, Ding S, Wyman CE (2011) Enzymatic hydrolysis of cellulosic biomass. Biofuels 2:421–449. https://doi.org/10.4155/bfs.11.116
Yildiz S, Gümüşkaya E (2007) The effects of thermal modification on crystalline structure of cellulose in soft and hardwood. Build Environ 42:62–67. https://doi.org/10.1016/j.buildenv.2005.07.009
Yu Q, Zhuang X, Zhenhong Y, Qiong Q, Wei Q, Wang W, Zhang Y, Xu J, Huijuan X (2010) Two-step liquid hot water pretreatment of Eucalyptus grandis to enhance sugar recovery and enzymatic digestibility of cellulose. Bioresour Technol 101:4895–4899. https://doi.org/10.1016/j.biortech.2009.11.051
Zawadzki J, Gawron J, Antczak A, Kłosińska T, Radomski A (2016) The influence of heat treatment on the physico-chemical properties of pinewood (Pinus sylvestris L.). Drewno 59:49–57. https://doi.org/10.12841/wood.1644-3985.135.04
Zhu L, O’Dwyer JP, Chang VS, Granda CB, Holtzapple MT (2008) Structural features affecting biomass enzymatic digestibility. Bioresour Technol 99:3817–3828. https://doi.org/10.1016/j.biortech.2007.07.033
Acknowledgements
This work was financed by a research project from the National Center for Research and Development, which was “Intelligent systems for breeding and cultivation of wheat, maize, and poplar for optimized biomass production, biofuels, and modified wood” (BIOSTRATEG2/298241/10/NCBR/2016). Poplar material used in presented work was obtained in Welcome 2008/1 project of the Foundation for Polish Science given to Prof. Stanisław Karpiński.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The article does not contain any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krutul, D., Szadkowski, J., Výbohová, E. et al. Effect of steam explosion pretreatment on chosen saccharides yield and cellulose structure from fast-growing poplar (Populus deltoides × maximowiczii) wood. Wood Sci Technol 58, 441–458 (2024). https://doi.org/10.1007/s00226-024-01532-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-024-01532-7