Abstract
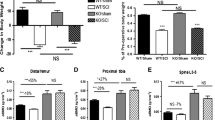
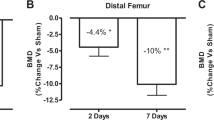
To date, no efficacious therapy exists that will prevent or treat the severe osteoporosis in individuals with neurologically motor-complete spinal cord injury (SCI). Recent preclinical studies have demonstrated that sclerostin antibody (Scl-Ab) can prevent sublesional bone loss after acute SCI in rats. However, it remains unknown whether sclerostin inhibition reverses substantial bone loss in the vast majority of the SCI population who have been injured for several years. This preclinical study tested the efficacy of Scl-Ab to reverse the bone loss that has occurred in a rodent model after chronic motor-complete SCI. Male Wistar rats underwent either complete spinal cord transection or only laminectomy. Twelve weeks after SCI, the rats were treated with Scl-Ab at 25 mg/kg/week or vehicle for 8 weeks. In the SCI group that did not receive Scl-Ab, 20 weeks of SCI resulted in a significant reduction of bone mineral density (BMD) and estimated bone strength, and deterioration of bone structure at the distal femoral metaphysis. Treatment with Scl-Ab largely restored BMD, bone structure, and bone mechanical strength. Histomorphometric analysis showed that Scl-Ab increased bone formation in animals with chronic SCI. In ex vivo cultures of bone marrow cells, Scl-Ab inhibited osteoclastogenesis, and promoted osteoblastogenesis accompanied by increased Tcf7, ENC1, and the OPG/RANKL ratio expression, and decreased SOST expression. Our findings demonstrate for the first time that Scl-Ab reverses the sublesional bone loss when therapy is begun after relatively prolonged spinal cord transection. The study suggests that, in addition to being a treatment option to prevent bone loss after acute SCI, sclerostin antagonism may be a valid clinical approach to reverse the severe bone loss that invariably occurs in patients with chronic SCI.






Similar content being viewed by others
Abbreviations
- SCI:
-
Spinal cord injury
- SOST :
-
Sclerostin
- Scl-Ab:
-
Sclerostin antibody
- BMD:
-
Bone mineral density
- MOI:
-
Moment of inertia
- FEA:
-
Finite element analysis
- BFR:
-
Bone formation rate
- MS/BS:
-
Mineralizing surface/bone surface
- DXA:
-
Dual-energy X-ray absorptiometer
- PFA:
-
Paraformaldehyde
- CFU-F:
-
Colony-forming unit-fibroblastic
- CFU-ob:
-
Colony-forming unit-osteoblastic staining
- MSC:
-
Mesenchymal stem cells
- TRAP:
-
Tartrate-resistant acid phosphatase
- H&E:
-
Hematoxylin and eosin
- CTX:
-
Serum C-terminal telopeptide of Type I collagen
- CTR:
-
Calcitonin receptor
- BV:
-
Bone volume
- TV:
-
Tissue volume
- Tb.N:
-
Trabecular number
- Tb.Th:
-
Trabecular thickness
- Tb.Sp:
-
Trabecular separation
- Conn.D:
-
Connectivity density
- SMI:
-
Structure model index
- OV:
-
Osteoid volume
- OS:
-
Osteoid surface
- N. Oc:
-
Osteoclast number
- Pm:
-
Perimeter
References
Bauman WA, Cardozo CP (2015) Osteoporosis in individuals with spinal cord injury. PM R 7(2):188–201. https://doi.org/10.1016/j.pmrj.2014.08.948
Qin W, Bauman WA, Cardozo C (2010) Bone and muscle loss after spinal cord injury: organ interactions. Ann N Y Acad Sci 1211:66–84. https://doi.org/10.1111/j.1749-6632.2010.05806.x
Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D et al (2009) Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int 20(3):385–92. https://doi.org/10.1007/s00198-008-0671-6
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42(4):606–615. https://doi.org/10.1016/j.bone.2007.12.224
Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H et al (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8(5):751–64. https://doi.org/10.1016/j.devcel.2005.02.017
Manolagas SC (2014) Wnt signaling and osteoporosis. Maturitas 78(3):233–237. https://doi.org/10.1016/j.maturitas.2014.04.013
Zaidi M (2007) Skeletal remodeling in health and disease. Nat Med 13(7):791–801. https://doi.org/10.1038/nm1593
Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR et al (2012) Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res 27(2):352–359. https://doi.org/10.1002/jbmr.546
Morse LR, Sudhakar S, Lazzari AA, Tun C, Garshick E, Zafonte R et al (2013) Sclerostin: a candidate biomarker of SCI-induced osteoporosis. Osteoporos Int 24(3):961–968. https://doi.org/10.1007/s00198-012-2072-0
Qin W, Li X, Peng Y, Harlow LM, Ren Y, Wu Y et al (2015) Sclerostin antibody preserves the morphology and structure of osteocytes and blocks the severe skeletal deterioration after motor-complete spinal cord injury in rats. J Bone Miner Res 30(11):1994–2004. https://doi.org/10.1002/jbmr.2549
Beggs LA, Ye F, Ghosh P, Beck DT, Conover CF, Balaez A et al (2015) Sclerostin inhibition prevents spinal cord injury-induced cancellous bone loss. J Bone Miner Res 30(4):681–689. https://doi.org/10.1002/jbmr.2396
Qin W, Zhao W, Li X, Peng Y, Harlow LM, Li J et al (2016) Mice with sclerostin gene deletion are resistant to the severe sublesional bone loss induced by spinal cord injury. Osteoporos Int. https://doi.org/10.1007/s00198-016-3700-x
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M et al (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10(5):537–43
Staehling-Hampton K, Proll S, Paeper BW, Zhao L, Charmley P, Brown A et al (2002) A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet 110(2):144–52. https://doi.org/10.1002/ajmg.10401
Semenov M, Tamai K, He X (2005) SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280(29):26770–26775. https://doi.org/10.1074/jbc.M504308200
Semenov MV, He X (2006) LRP5 mutations linked to high bone mass diseases cause reduced LRP5 binding and inhibition by SOST. J Biol Chem 281(50):38276–38284. https://doi.org/10.1074/jbc.M609509200
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D et al (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23(6):860–869. https://doi.org/10.1359/jbmr.080216
MacDonald BT, Joiner DM, Oyserman SM, Sharma P, Goldstein SA, He X et al (2007) Bone mass is inversely proportional to Dkk1 levels in mice. Bone 41(3):331–339. https://doi.org/10.1016/j.bone.2007.05.009
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I et al (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283(9):5866–5875. https://doi.org/10.1074/jbc.M705092200
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J et al (2009) Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 24(10):1651–1661. https://doi.org/10.1359/jbmr.090411
Sun L, Pan J, Peng Y, Wu Y, Li J, Liu X et al (2013) Anabolic steroids reduce spinal cord injury-related bone loss in rats associated with increased Wnt signaling. J Spinal Cord Med 36(6):616–22. https://doi.org/10.1179/2045772312Y.0000000020
Qin W, Sun L, Cao J, Peng Y, Collier L, Wu Y et al (2013) The central nervous system (CNS)-independent anti-bone-resorptive activity of muscle contraction and the underlying molecular and cellular signatures. J Biol Chem 288(19):13511–13521. https://doi.org/10.1074/jbc.M113.454892
Jin Y, Fischer I, Tessler A, Houle JD (2002) Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol 177(1):265–75
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39(5):777–87
Fischer FR, Peduzzi JD (2007) Functional recovery in rats with chronic spinal cord injuries after exposure to an enriched environment. J Spinal Cord Med 30(2):147–55
Wang X, Duffy P, McGee AW, Hasan O, Gould G, Tu N et al (2011) Recovery from chronic spinal cord contusion after Nogo receptor intervention. Ann Neurol 70(5):805–21. https://doi.org/10.1002/ana.22527
Hesp ZC, Goldstein EZ, Miranda CJ, Kaspar BK, McTigue DM (2015) Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J Neurosci 35(3):1274–1290. https://doi.org/10.1523/JNEUROSCI.2568-14.2015
Ruth EB. Metamorphosis of the pubic symphysis. I. The white rat (Mus norvegicus albinus). Anat Rec. 1935;(64):1–7
Voor MJ, Brown EH, Xu Q, Waddell SW, Burden RL, Burke DA et al (2011) Bone loss following spinal cord injury in a rat model. J Neurotrauma. https://doi.org/10.1089/neu.2011.2037
Minematsu A, Nishii Y, Imagita H, Sakata S (2014) Time course of changes in trabecular bone microstructure in rats with spinal cord injury. J Life Sci. 8(6):522–528
Zamarioli A, Battaglino RA, Morse LR, Sudhakar S, Maranho DA, Okubo R et al (2013) Standing frame and electrical stimulation therapies partially preserve bone strength in a rodent model of acute spinal cord injury. Am J Phys Med Rehabil 92(5):402–10. https://doi.org/10.1097/PHM.0b013e318287697c
Lin T, Tong W, Chandra A, Hsu SY, Jia H, Zhu J et al (2015) A comprehensive study of long-term skeletal changes after spinal cord injury in adult rats. Bone Res 3:15028. https://doi.org/10.1038/boneres.2015.28
Ren Y, Han X, Ho SP, Harris SE, Cao Z, Economides AN et al (2015) Removal of SOST or blocking its product sclerostin rescues defects in the periodontitis mouse model. FASEB J 29(7):2702–2711. https://doi.org/10.1096/fj.14-265496
Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J et al (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24(4):578–588. https://doi.org/10.1359/jbmr.081206
Chen H, Xu X, Liu M, Zhang W, Ke HZ, Qin A et al (2015) Sclerostin antibody treatment causes greater alveolar crest height and bone mass in an ovariectomized rat model of localized periodontitis. Bone 76:141–148. https://doi.org/10.1016/j.bone.2015.04.002
Taut AD, Jin Q, Chung JH, Galindo-Moreno P, Yi ES, Sugai JV et al (2013) Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J Bone Miner Res 28(11):2347–2356. https://doi.org/10.1002/jbmr.1984
Bramlett HM, Dietrich WD, Marcillo A, Mawhinney LJ, Furones-Alonso O, Bregy A et al (2014) Effects of low intensity vibration on bone and muscle in rats with spinal cord injury. Osteoporos Int 25(9):2209–2219. https://doi.org/10.1007/s00198-014-2748-8
Cardozo CP, Qin W, Peng Y, Liu X, Wu Y, Pan J et al (2010) Nandrolone slows hindlimb bone loss in a rat model of bone loss due to denervation. Ann N Y Acad Sci 1192:303–306. https://doi.org/10.1111/j.1749-6632.2009.05313.x
Pistoia W, van Rietbergen B, Lochmuller EM, Lill CA, Eckstein F, Ruegsegger P (2002) Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone 30(6):842–848
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Battaglino RA, Sudhakar S, Lazzari AA, Garshick E, Zafonte R, Morse LR (2012) Circulating sclerostin is elevated in short-term and reduced in long-term SCI. Bone 51(3):600–605. https://doi.org/10.1016/j.bone.2012.04.019
Invernizzi M, Carda S, Rizzi M, Grana E, Squarzanti DF, Cisari C et al (2015) Evaluation of serum myostatin and sclerostin levels in chronic spinal cord injured patients. Spinal Cord 53(8):615–620. https://doi.org/10.1038/sc.2015.61
Shahnazari M, Wronski T, Chu V, Williams A, Leeper A, Stolina M et al (2012) Early response of bone marrow osteoprogenitors to skeletal unloading and sclerostin antibody. Calcif Tissue Int 91(1):50–58. https://doi.org/10.1007/s00223-012-9610-9
Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ et al (2013) Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res 28(4):865–74. https://doi.org/10.1002/jbmr.1807
Tian X, Jee WS, Li X, Paszty C, Ke HZ (2011) Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone 48(2):197–201. https://doi.org/10.1016/j.bone.2010.09.009
Holdsworth G, Greenslade K, Jose J, Stencel Z, Kirby H, Moore A et al (2018) Dampening of the bone formation response following repeat dosing with sclerostin antibody in mice is associated with up-regulation of Wnt antagonists. Bone 107:93–103. https://doi.org/10.1016/j.bone.2017.11.003
Taylor S, Ominsky MS, Hu R, Pacheco E, He YD, Brown DL et al (2016) Time-dependent cellular and transcriptional changes in the osteoblast lineage associated with sclerostin antibody treatment in ovariectomized rats. Bone 84:148–59. https://doi.org/10.1016/j.bone.2015.12.013
Florio M, Gunasekaran K, Stolina M, Li X, Liu L, Tipton B et al (2016) A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun 7:11505. https://doi.org/10.1038/ncomms11505
Chang KV, Hung CY, Chen WS, Lai MS, Chien KL, Han DS (2013) Effectiveness of bisphosphonate analogues and functional electrical stimulation on attenuating post-injury osteoporosis in spinal cord injury patients- a systematic review and meta-analysis. PLoS ONE 8(11):e81124. https://doi.org/10.1371/journal.pone.0081124
Acknowledgements
This work was supported by the Veterans Health Administration, Rehabilitation Research and Development Service (Grants 5I01RX001313, 5I01RX02089-A2, and 5I01RX000687 to WQ; B9212-C and B2020-C to WAB). Amgen Inc. and UCB Pharma provided Scl-Ab.
Author information
Authors and Affiliations
Contributions
WZ, XL, HK, WAB, and WQ were responsible for study design and data analysis. WZ, XL, YP, JP, JL, AX, YQ, JF, and CPC conducted the study. The manuscript was written by WZ and WQ and was revised and approved by all authors. WQ takes responsibility for the integrity of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
Wei Zhao, Yuanzhen Peng, Yiwen Qin, Jianping Pan, Jiliang Li, Aihua Xu, Jian Q. Feng, William A. Bauman, Christopher Cardozo, and Weiping Qin declare that they have no conflict of interest. XL is current employee and shareholder of Amgen Inc. MSO is an ex-Amgen employee and owns Amgen stocks, and HZK is current employee of UCB Pharma and shareholder of UCB Pharma and Amgen.
Human and Animal Rights and Informed Consent
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, W., Li, X., Peng, Y. et al. Sclerostin Antibody Reverses the Severe Sublesional Bone Loss in Rats After Chronic Spinal Cord Injury. Calcif Tissue Int 103, 443–454 (2018). https://doi.org/10.1007/s00223-018-0439-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0439-8