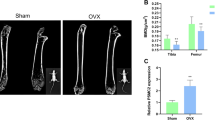
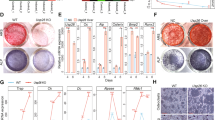
Abstract
Osteoporosis is a common disease that increases individual’s fragility fracture risk. PTH is the only therapeutic agent for severe osteoporosis that requires anabolic action of bone formation. Although a part of the PTH actions is explained by increased proliferation of osteoblastic precursor cells, the mechanisms involved in the proliferation of osteoblastic cells by PTH have not been clarified yet. Therefore, in this study, we investigated the effects of PTH on gene expression in the cultured osteoblastic MC3T3-E1 cells, and found that the ubiquitin-specific peptidase 2 (Usp2) may be one of the direct target genes of PTHR signaling. Usp2 is a deubiquitination enzyme targeting various factors including CyclinD1 in cancer cells and PTH receptor 1 in osteoblasts. We confirmed that consistent induction of Usp2 expression peaked at 1 h by PTH1-34 (teriparatide) in MC3T3-E1 cells and primary calvarial osteoblasts. Among the three known splicing variants of the Usp2, we found the isoforms 1 and 2 are predominantly expressed in osteoblasts. Live-imaging analysis of the Fucci-transgenic mouse-derived primary osteoblasts indeed demonstrated that Usp2 is required for the PTH1-34-induced osteoblast proliferation. Western blotting analysis of the CyclinD1 indicated that Usp2 knock-down influences the paradoxical changes of CyclinD1 protein levels in this condition. Our data indicate that Usp2 is required for the PTH1-34-induced proliferation of osteoblasts.





Similar content being viewed by others
References
Armas LA, Recker RR (2012) Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol Metab Clin North Am 41(3):475–486
Gerdhem P (2013) Osteoporosis and fragility fractures: vertebral fractures. Best Pract Res Clin Rheumatol 27(6):743–755
Lim SY, Bolster MB (2015) Current approaches to osteoporosis treatment. Curr Opin Rheumatol 27(3):216–224
Silva BC, Bilezikian JP (2015) Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol 22:41–50
Jilka RL (2007) Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40(6):1434–1446
Saini V, Marengi DA, Barry KJ, Fulzele KS, Heiden E, Liu X, Dedic C, Maeda A, Lotinun S, Baron R, Pajevic PD (2013) Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem 288(28):20122–20134
Datta NS, Abou-Samra AB (2009) PTH and PTHrP signaling in osteoblasts. Cell Signal 21(8):1245–1254
Moriya S, Hayata T, Notomi T, Aryal S, Nakamaoto T, Izu Y, Kawasaki M, Yamada T, Shirakawa J, Kaneko K, Ezura Y, Noda M (2015) PTH regulates β2-adrenergic receptor expression in osteoblast-like MC3T3-E1 cells. J Cell Biochem 116(1):142–148
Silvestrini G, Ballanti P, Leopizzi M, Sebastiani M, Berni S, Di Vito M, Bonucci E (2007) Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J Mol Histol 38(4):261–269
Linkhart TA, Mohan S (1989) Parathyroid hormone stimulates release of insulin-like growth factor-I (IGF-I) and IGF-II from neonatal mouse calvaria in organ culture. Endocrinology 125(3):1484–1491
Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S (2001) Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology 142(10):4349–4356
Bikle DD, Wang Y (2012) Insulin like growth factor-I: a critical mediator of the skeletal response to parathyroid hormone. Curr Mol Pharmacol 5(2):135–142
Tian Y, Xu Y, Fu Q, He M (2011) Parathyroid hormone regulates osteoblast differentiation in a Wnt/β-catenin-dependent manner. Mol Cell Biochem 355(1–2):211–216
Matsumoto T, Kuriwaka-Kido R, Kondo T, Endo I, Kido S (2012) Regulation of osteoblast differentiation by interleukin-11 via AP-1 and Smad signaling. Endocr J 59(2):91–101
Kuriwaka-Kido R, Kido S, Miyatani Y, Ito Y, Kondo T, Omatsu T, Dong B, Endo I, Miyamoto K, Matsumoto T (2013) Parathyroid hormone (1-34) counteracts the suppression of interleukin-11 expression by glucocorticoid in murine osteoblasts: a possible mechanism for stimulating osteoblast differentiation against glucocorticoid excess. Endocrinology 154(3):1156–1167
Datta NS, Pettway GJ, Chen C, Koh AJ, McCauley LK (2007) Cyclin D1 as a target for the proliferative effects of PTH and PTHrP in early osteoblastic cells. J Bone Miner Res 22(7):951–964
Shirakawa J, Ezura Y, Moriya S, Kawasaki M, Yamada T, Notomi T, Nakamoto T, Hayata T, Miyawaki A, Omura K, Noda M (2014) Migration linked to FUCCI-indicated cell cycle is controlled by PTH and mechanical stress. J Cell Physiol 229(10):1353–1358
Wang YH, Liu Y, Rowe DW (2007) Effects of transient PTH on early proliferation, apoptosis, and subsequent differentiation of osteoblast in primary osteoblast cultures. Am J Physiol Endocrinol Metab 292(2):E594–E603
Charest-Morin X, Fortin JP, Lodge R, Allaeys I, Poubelle PE, Marceau F (2014) A tagged parathyroid hormone derivative as a carrier of antibody cargoes transported by the G protein coupled PTH1 receptor. Peptides 60:71–79
Lewinson D, Rachmiel A, Rihani-Bisharat S, Kraiem Z, Schenzer P, Korem S, Rabinovich Y (2003) Stimulation of Fos- and Jun-related genes during distraction osteogenesis. J Histochem Cytochem 51(9):1161–1168
Matsuda N, Morita N, Matsuda K, Watanabe M (1998) Proliferation and differentiation of human osteoblastic cells associated with differential activation of MAP kinases in response to epidermal growth factor, hypoxia, and mechanical stress in vitro. Biochem Biophys Res Commun 249(2):350–354
Miles RR, Sluka JP, Halladay DL, Santerre RF, Hale LV, Bloem L, Patanjali SR, Galvin RJ, Ma L, Hock JM, Onyia JE (2002) Parathyroid hormone (hPTH 1-38) stimulates the expression of UBP41, an ubiquitin-specific protease, in bone. J Cell Biochem 85(2):229–242
Pouly D, Debonneville A, Ruffieux-Daidié D, Maillard M, Abriel H, Loffing J, Staub O (2013) Mice carrying ubiquitin-specific protease 2 (Usp2) gene inactivation maintain normal sodium balance and blood pressure. Am J Physiol Renal Physiol 305(1):F21–F30
Bedard N, Yang Y, Gregory M, Cyr DG, Suzuki J, Yu X, Chian RC, Hermo L, O’Flaherty C, Smith CE, Clarke HJ, Wing SS (2011) Mice lacking the USP2 deubiquitinating enzyme have severe male subfertility associated with defects in fertilization and sperm motility. Biol Reprod 85(3):594–604
Alonso V, Magyar CE, Wang B, Bisello A, Friedman PA (2011) Ubiquitination-deubiquitination balance dictates ligand-stimulated PTHR sorting. J Bone Miner Res 26(12):2923–2934
Shan J, Zhao W, Gu W (2009) Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol Cell 36(3):469–476
Stevenson LF, Sparks A, Allende-Vega N, Xirodimas DP, Lane DP, Saville MK (2007) The deubiquitinating enzyme USP2a regulates the p53 pathway by targeting Mdm2. EMBO J 26(4):976–986
Wang CL, Wang JY, Liu ZY, Ma XM, Wang XW, Jin H, Zhang XP, Fu D, Hou LJ, Lu YC (2014) Ubiquitin-specific protease 2a stabilizes MDM4 and facilitates the p53-mediated intrinsic apoptotic pathway in glioblastoma. Carcinogenesis 35(7):1500–1509
Park KC, Kim JH, Choi EJ, Min SW, Rhee S, Baek SH, Chung SS, Bang O, Park D, Chiba T, Tanaka K, Chung CH (2002) Antagonistic regulation of myogenesis by two deubiquitinating enzymes, UBP45 and UBP69. Proc Natl Acad Sci USA 99(15):9733–9738
Gousseva N, Baker RT (2003) Gene structure, alternate splicing, tissue distribution, cellular localization, and developmental expression pattern of mouse deubiquitinating enzyme isoforms Usp2-45 and Usp2-69. Gene Expr 11(3–4):163–179
Guo Y, Stacey DW, Hitomi M (2002) Post-transcriptional regulation of cyclin D1 expression during G2 phase. Oncogene 21(49):7545–7556
Stacey DW (2003) Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol 15(2):158–163
Stacey DW, Hitomi M (2008) Cell cycle studies based upon quantitative image analysis. Cytometry A 73(4):270–278
Acknowledgments
This study was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan Aerospace Exploration Agency (JAXA), and The Tokyo Biochemical Research Foundation (TBRF). The authors thank all the staff members in the Stem Cell Laboratory of Medical Research Institute in Tokyo Medical and Dental University for valuable helps and suggestions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Jumpei Shirakawa, Hiroyuki Harada, Masaki Noda, and Yoichi Ezura declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The animal experiments were conducted according to the institutional guidelines for animal welfare, and were approved by the institutional committee (approval number: 0070003).
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shirakawa, J., Harada, H., Noda, M. et al. PTH-Induced Osteoblast Proliferation Requires Upregulation of the Ubiquitin-Specific Peptidase 2 (Usp2) Expression. Calcif Tissue Int 98, 306–315 (2016). https://doi.org/10.1007/s00223-015-0083-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0083-5