Abstract
Quinoa is a highly nutritious crop with diverse applications in the food industry. The study assessed the impact of various processing techniques, including microwaving, boiling, roasting, steaming, flaking, and germination, on the crude protein content (CP), total phenolic content (TPC), antioxidant activity (AA), and 12 phenolic compounds in quinoa. CP was significantly affected by the heat treatments. Boiled quinoa flakes exhibited the highest average CP, boiling and roasting the lowest. Microwaving strongly enhanced the TPC and the content of six bioactive compounds (CFA, KMP, NAR, QCE, RUT, SA), while boiling and steaming had the most adverse effect. Germination improved the overall nutritional profile of quinoa. The most pronounced increase in the bioactive metabolites occurred between the third and fifth day of germination in a genotype-dependent manner. Six metabolites (NAR, SA, 4BA, IQ, PC, IH) were detected in germinated quinoa for the first time. The results emphasize the substantial influence of processing techniques and type of sample on quinoa nutritional quality and underscore the importance of proper consideration of those factors to obtain nutritionally optimal food products.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Quinoa (Chenopodium quinoa Willd.) is a highly versatile crop with outstanding nutritional value, which was recognized even by ancient Andean populations, considering this pseudocereal a sacred food [1]. Although its cultivation has already spread worldwide, the biggest producers are still the countries of quinoa origin—Peru, Bolivia, and Ecuador [2]. Quinoa has been traditionally consumed in a form of grain or as an ingredient in many food products and dishes, such as soups, porridges, buns, and drinks [3]. Its growing popularity has led to the development of novel foods containing quinoa, in particular gluten-free, vegetarian, vegan, and dairy-free products [4, 5].
Although the quinoa’s nutrient-rich profile has been a subject of extensive research, most of the studies were, however, realized on raw materials. Nonetheless, quinoa is usually not eaten raw but processed in order to decrease the content of anti-nutritional compounds, such as saponins and phytic acid [6, 7].Studied have shown that commonly used processing methods, such as boiling, steaming, microwaving, and extrusion may alter the nutritional content and composition, as well as the overall bioavailability of nutrients. For example, microwaving and boiling under pressure have been reported as a suitable technique for preservation of polyphenols. In comparison, boiling caused the major loss of phenolic compounds and minerals [6, 8]. Although the protein content is not significantly affected by the common heat-utilizing preparations [6], it has been described that some methods like microwaving and fermentation may increase the protein digestibility of final quinoa product [9, 10].
Apart from heat-utilizing preparations, germination has emerged as an alternative and relatively cheap processing technique for improving the nutritional profile by promoting enzymatic activity and release of various bioactive chemical sand minerals [11, 12], while reducing the content of anti-nutritional factors like phytic acid and tannin [13, 14]. Germination may further improve biological value of quinoa protein and its overall digestibility [15, 16].
Comprehensive studies are needed to explore the impact of different preparation methods on those traits and their implications for further food processing. Therefore, the aim of this paper was to evaluate a spectrum of quinoa seed preparation methods and evaluate their impact on the content of protein, antioxidant activity, total phenolic content and 13 phenolic compounds. By providing an extensive analysis of these effects, this paper aims to raise the awareness around quinoa nutritional quality and the selection of appropriate processing techniques preserving the high-quality nutritional profile of quinoa food products.
Materials and methods
Plant material
A total of three quinoa samples were subjected to analysis. The original seeds of two quinoa samples (genotypes ‘Besançon’ and ‘Faro’) were provided from the U.S. National Plant Germplasm System operated by USDA. The seeds of these two genotypes were multiplied to provide sufficient material for further experiments in the experimental field of the Crop Research Institute in Prague in the Czech Republic during the year 2021. One commercial quinoa sample (Probio) was kindly provided by PRO-BIO Ltd, Czech Republic.
Procedure of germination
Germination was carried out on commercial Probio sample and genotypes ‘Besançon’ and ‘Faro’. A total of 10 g of healthy and undamaged seeds of each sample was used for the experiment. In addition, seeds of genotypes ‘Besançon’ and ‘Faro’ were thoroughly rinsed in 30% (v/v) hydrogen peroxide for disinfection purposes to minimize microbiological contamination of the seed surface from the field condition. Then, seeds of all three samples were washed several times in distilled water. All three samples were soaked in distilled water for 4 h, drained and then placed in a sterile Petri dish lined with moist filter paper and covered with the lid. Hydrated quinoa seeds were allowed to germinate for 1 day (24 h), 2 days (48 h), 3 days (72 h), 4 days (96 h), and 5 days (120 h), respectively. Germination of Probio sample is shown in Fig. 1. Germination was performed at the room temperature under a 16/8 day/night regime and seeds were regularly watered with distilled water. Sprouted seeds were collected each day of germination and lyophilized before next use. Samples were stored in cold and dark place for following laboratory analyses. The non-germinated samples were indicated as control samples.
Processing techniques
The commercial sample Probio was subjected to several heat-utilizing processing techniques. All processing techniques were carried out under atmospheric pressure in room temperature. Prior to each thermal processing, Probio sample was soaked in distilled water for 24 h. The excess water was drained from the samples prior following treatments. For each treatment, the sampling intervals were established. After each sampling interval, quinoa seeds were immediately drained from any excess water, transferred to a sterile container, and labeled accordingly for subsequent analysis. After a cool-down, samples were lyophilized and stored in cold and dark place for following laboratory analyses. The non-processed samples were indicated as control samples.
In the case of boiling in plain water, grains were boiled in distilled water in the ratio of 1:2 (w/v) for 5, 10, and 15 min. The boiling in NaCl used the same proceeding, salt was at the concertation of 10 g/L (w/v). Microwaving was realized in the microwave oven (ETA 2209 90,000, ETA a.s., Czech Republic) for 1, 2, and 3 min at the power of 1050 W. Another batch of samples was roasted on the pan for 5, 10, and 15 min at the temperature of 180 ± 20 °C. Lastly, steaming was carried out by placing the quinoa grains on a fine mesh sieve and set over boiling water, covered with a lid. The sample was steamed for 5, 10, and 15 min.
Further, raw Probio seeds were mechanically pressed to obtain flakes using a food processor (Jupiter Küchenmaschinen, System Drive Unit, Weimar, Germany) equipped with a flake roller. Raw quinoa flakes were boiled in distilled water in the ratio of 1:2 (w/v) for 1, 2, 3, 4, and 5 min following the same procedure as mentioned in the first paragraph. Boiled flakes were subjected to chemical analysis.
Chemicals
Polyphenolic compounds, including 4-OH benzaldehyde, caffeic acid, gallic acid, isoquercetin, isorhamnetin, kaempferol, naringenin, pinocembrin, quercetin, quercetin 3-O-glucuronide, rutin, and salicylic acid, along with the internal standard probenecid were procured from Sigma–Aldrich (St. Louis, MO, USA). Methanol of LC–MS grade (≥ 99.9%) was sourced from Riedel de Haën (Seelze, Germany), while formic acid of LC–MS grade (99%) was obtained from VWR (Leuven, Belgium). Pure water was acquired from a Milli-Q purification system manufactured by Millipore (Bedford, MA, USA).
Standard and sample preparation
The preparation of reference stock solutions involved dissolving the methanol-dissolved reference standards of each phenolic compound to create stock solutions at a concentration of 0.5 mg/mL. These reference stock solutions were subsequently stored at − 18 °C. To establish the calibration curves for quantifying the phenolic compounds, the stock solutions were diluted within a methanol to concentration range of 0.001–2.000 μg/mL. In addition, probenecid was dissolved in methanol at a concentration of 0.5 mg/mL to generate a stock solution of the internal standard. The internal standard was then added to the individual reference standard solutions or test samples, resulting in a final concentration of 0.1 μg/mL.
For the analysis using mass spectrometry, the lyophilized samples were milled using an IKA A11 basic mill (IKA-Werke, Staufen, Germany), and the resulting mixture was stored in well-sealed plastic bags in a dark, cold place at 4 °C. The extraction of sample followed the method described by Janovská et al. [17]. Briefly, 0.1 g of the milled mixture was extracted twice with 1 mL of extraction solvent (comprising 80% methanol with probenecid as internal standard at a concentration of 0.1 µg/mL) in Eppendorf tubes. The extraction was performed using an ultrasonic bath for 60 min at 45 °C. After extraction, the samples were centrifuged for 10 min at 13,500 rpm. The obtained supernatants from each sample were then filtered through 0.2 µm nylon syringe filters for further analysis.
UHPLC-ESI–MS/MS instrumentation
The chromatographic analysis was conducted using the Dionex UltiMate 3000 UHPLC system (Dionex Softron GmbH, Germering, Germany), comprising a binary pump (HPG-3400RS), an autosampler (WPS-3000RS), a degasser (SRD-3400), and a column oven (TCC-3000RS). Detection of analytes was performed on the quadrupole/orbital ion trap Q Exactive mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA). The LC–MS system was equipped with a heated electrospray ionization source (HESI-II) and operated using Xcalibur software, version 4.0 (Thermo Fisher Scientific, San Jose, CA, USA).
UHPLC-ESI–MS/MS analysis
The analytes were separated on a reversed-phase C18 Ascentis Express column (2.1 × 100 mm, 2.7 μm) from Supelco (Bellefonte, PA, USA). The chromatographic separation was performed using a gradient elution method. Solvent A consisted of 0.2% formic acid (v/v) in water, while solvent B comprised methanol with 0.2% formic acid (v/v). The LC gradient commenced with 99% of solvent A and 1% of solvent B at 0 min., followed by a linear gradient elution to 40% A and 60% B at 11 min. The column was then flushed with 100% solvent B for 2 min. Equilibration of the column was accomplished by washing with 99% A and 1% B for additional 2 min. The total analysis time was 15 min. The column temperature was maintained at 40 °C, and the flow rate was set to 0.35 mL/min. The injection volume was 1 µL.
The mass spectrometer analysis was conducted in negative electrospray ionization (ESI) mode. The spray voltage was set at − 2.5 kV, and the sheath gas flow rate, auxiliary gas flow rate, and sweep gas flow rate were 49, 12, and 2 arbitrary units, respectively. The capillary temperature was 260 °C, and nitrogen was used as the sheath, auxiliary, and sweep gas. The heater temperature was maintained at 419 °C, and the S-lens RF level was set to 30. Precursor ions in the inclusion list were isolated within a retention time window of ± 60 s, filtered in the quadrupole at the isolation window (target m/z ± 0.8 m/z), and fragmented in an HCD collision cell C-trap at a resolution of 17,500 FWHM (full width at half maximum). The AGC target value was 1 × 106, and the maximum injection time was 50 ms.
The normalized collision energy (NCE) was optimized for each compound. Details of the precursor and daughter ions monitored, retention times, and NCE values can be found in Table S1. The precision and calibration of the Q Exactive Orbitrap LC/MS/MS instrument were assessed using a reference standard mixture provided by Thermo Fisher Scientific. The measurements were performed in triplicate, and the data were evaluated using Quan/Qual Browser Xcalibur software, version 4.0.
Determination of the phenolic compound concentration in quinoa samples
The identification of phenolic compounds in the quinoa samples relied on their retention times compared to authentic standards and the analysis of mass spectral data obtained through LC–MS/MS. Accurate mass determination was employed to generate elemental compositions and fragmentation patterns of the molecular ions. Quantification was done on the basis of the transition from precursor ion [M + H]+ to corresponding quantification ion (Table S1). Calibration curves were then established by plotting the peak area, adjusted with probenecid as internal standard, against the concentration of the corresponding reference standards.
Chemical analyses
All three quinoa samples were also investigated for the effects of germination on the protein content (CP), total phenolic content (TPC), and antioxidant activity (AA). The CP content of each sample was measured using the classical Kjeldahl mineralization method and calculated using a conversion factor of 6.25 [18]. For this method, 1 g of milled sample was utilized. The TPC was determined using Folin–Ciocalteau (FC) reagent with slight modifications based on the method [19]. The FC method employed 2 g of sample. The TPC results were expressed as grams of gallic acid equivalent (GAE) per kilogram of sample dw (GAE g/kg dw). The AA of the samples was assessed using a DPPH assay [20], utilized 1 g of milled sample in this study. The results of the DPPH assay were expressed as millimoles of Trolox equivalent (TE) per gram of sample dw (μmol TE/g dw). Two replicates were performed for each protein content, TPC, and AA measurement.
Statistical analyses
Three biological replicates were measured for descriptors of interest. Statistical analysis was conducted using the R program [21]. Means and standard deviations were calculated for each sample type and processing method in individual traits. One-way analysis of variance (ANOVA) was performed to determine whether there was significant effect of preparation method or sample type on evaluated traits. For germination data, the method was also applied to confirm if there is significant difference between three evaluated cultivars. Tukey’s honestly significant difference (HSD) test was employed to identify processing methods and its variants with significantly different means. To explore the association among samples, a principal component analysis (PCA) was conducted using scaled data for a set of 14 descriptors. Quality of representation of variables on the factor map was also assessed for the first two components with the largest variance. The routines within FactoMineR [22] and factoextra packages [23] were used for this task and to visualize PCA results.
Results and discussion
The effect of germination
The presented study investigated the effects of germination on the CP, TPC, AA, and the quantity of twelve metabolites in two quinoa genotypes ‘Faro’ and ‘Besançon’ and one commercial sample Probio (Fig. 2).Throughout the germination process, slight, but statistically significant differences (at p < 0.05) were reported for CP depending on the sample and the duration of germination. The major increase in CP was observed in the Probio sample on the fourth day of germination reaching 17.18 ± 0.01% dw in comparison to control (15.47 ± 0.21% dw). In ‘Faro’ and ‘Besançon’, the peak CP values were indicated on the third day of germination reaching 13.93 ± 0.02% and 15.58 ± 0.15% dw, respectively. The increase in CP has already been recognized not only in quinoa, but also in other related species from family Amaranthaceae [14]. This increase could be attributed to the enhanced enzymatic activity of α-amylase and the release of packed proteins from starch granules [24] or synthesis of proteins de novo [14]. Further, loss of total dry weight of the seeds due to the respiration during germination may increase the CP percentage [14].
The effect of germination time on selected nutritional parameters of three quinoa samples. Significant differences in means among control (C) and days of germination [1 day (G1D), 2 days (G2D), 3 days (G3D), 4 days (G4D), and 5 days (G5D)], are denoted by the different letters (Tukey HSD) above each column. Letters a-c indicates statistical differences within treatments, while letters A-E denotes statistical differences among treatments for individual cultivars. The abbreviations for the selected descriptors are as follows: gallic acid (GA), 4-hydroxybenzaldehyde (C4B), caffeic acid (CFA), quercetin-3-O-glucoronide (Q3G), isoquercetin (IQ), rutin (RUT), salicylic acid (SA), quercetin (QCE), naringenin (NAR), kaempferol (KMP), pinocembrin (PC), isorhamnetin (IH), crude protein content (CP), antioxidant activity (AA), total phenolic content (TPC)
Germination resulted as the superior technique for the enhanced AA in quinoa among other processing methods examined in this study. Although the initial days of germination resulted in a decline in AA values for the ‘Besançon’ and ‘Faro’ by almost 30%, the subsequent days of germination stimulated a continuous increase in this trait. The samples presented the peak values in AA during the third, fourth, and fifth day of germination in ‘Faro’, ‘Besançon’, and Probio, respectively. It was previously stated that the increase of AA is likely due to the elevated activity of hydrolytic enzymes and/or biosynthesis of antioxidants of low molecular weight [7], however, the level of increment is influenced by germination conditions [7]. Besides, our results indicated that differences in AA increment were related to the studied sample/genotype, confirming the earlier reported research carried out on white and red quinoa [25]. In our case, the most promising sample was Probio, since it did not show any remarkable drop in the beginning of germination, and it further reached the highest AA values on the fourth day of germination among other studied samples.
Significant variations (p < 0.05) in TPC were indicated among the quinoa samples and germination days. The highest TPC was recorded for the ‘Besançon’ (25.77 ± 0.15 GAE g/kg dw) on the second day of germination, which is aligns with the findings of Guardianelli et al. [25], but conflicting with Bhinder et al. [25], who recognized the peak values during the third and fourth day of germination. Detected contradictions may be attributed to the dynamic chemical changes during the germination including compound synthesis, release from bound form, or consumption [26]. In addition, specific germination conditions should be taken into consideration as factor influencing the TPC during germination [27]. As opposed to ‘Besançon’, ‘Faro,’ and Probio samples showcased their highest TPC values in the non-germinated state (23.71 ± 0.08 and 22.46 ± 0.88 GAE g/kg dw, respectively). Different rates of polyphenol accumulation in two different quinoa samples were published formerly [25], indicating the importance of proper genotype selection for obtaining the optimal quantity of phenolic compounds during germination.
The content of twelve studied metabolites as determined by UHPLC-ESI–MS/MS analysis is given in Fig. 2. The metabolite with the highest mean concentration in non-germinated samples was quercetin 3-O-glucuronide (Q3G), also known as miquelianin, whereas rutin (RUT) was the most abundant compound in germinated samples. RUT demonstrated an increasing accumulation with extended germination time, similar to what was presented in the study of Al-Qabba et al. [28] and Bhinder et al. [28]. The peak values of RUT content were recognized between the fourth and fifth day of germination but the degree of the increment varied among the samples. In our case, the most prominent increment in RUT content was reported for genotype ‘Faro’.
As mentioned in the beginning, Q3G was the most abundant metabolite in non-germinated quinoa seeds, which is in agreement with Dostalikova et al. [29]. This metabolite has been primarily detected in aerial plant parts in various plant species [30,31,32], but research quantifying the content of Q3G in seeds is insufficient. During the germination, Q3G showcased an opposite pattern as RUT with 80% decline in the initial days of germination in ‘Besançon’ and ‘Faro’. Contradictory results were published by Pilco-Quesada et al. [16] demonstrating a significant growth in the content of Q3G after 72 h of quinoa germination.
The isoquercetin (IQ) followed the same trend as discussed here in the case of Q3G. The drop in values was also noticed for salicylic acid (SA) and 4-hydroxybenzaldehyde (4BA) after first day of germination. Gallic acid (GA), naringenin (NAR), and caffeic acid (CFA) were presented in quinoa samples in a relatively trace concentrations, with respect to other studied compounds. The germination process improved their content, especially during the first 3 days of germination. To the best of our knowledge, NAR, SA, 4BA, and IQ have not been formerly quantified in germinated quinoa.
A small amount of kaempferol (KMP), pinocembrin (PC), quercetin (QCE), and isorhamnetin (IH) was detected in non-germinated samples. These metabolites were rapidly synthetized during the fifth day of the germination process, but the degree of increment varied among the studied genotypes. Besides, the mean PC content was the highest in germinated quinoa contrasting to raw and heat-treated samples. While the increase in KMP and QCE concentrations during germination has been already published for quinoa [7, 28], it was not as prominent as observed in our study. To our knowledge, the presence of PC and IH in germinated quinoa has not been evaluated before, nonetheless, they have already been described in sprouted mung bean [33] and buckwheat [34].
Overall, the germination process led to the enhancement of several bioactive compounds, including GA, CFA, RUT, QCE, NAR, KMP, PC, and IH in comparison to the control sample, suggesting their potential role in the germination process. The most substantial increase in the content of these metabolites was reported between the third and fifth days of germination. Conversely, germination initiated a decline in the levels of 4BA, Q3G, IQ, and SA. The alterations in metabolite quantity occurred in genotype-dependent manner, with ‘Besançon’ and ‘Faro’ exhibiting the most intense synthesis of metabolites during germination. On the other hand, the changes in chemical content of the Probio sample were less prominent.
It has been suggested previously that various metabolic and enzymatic events occurring during germination may synthesize or consume the phenolic compounds, thus elevating or decreasing their overall content. In addition, those compounds play a non-negligible role in protection against free radicals generated during the germination process [26]. However, other factors like genotype, agronomic conditions, maturity level at harvest and post-harvest storage conditions may considerably contribute to the variations in the polyphenol content of germinated quinoa [26].
Seed soaking
Quinoa seeds are prized for their superior nutritional quality, especially their high content of proteins and bioactive chemicals [35]. While this area has been researched extensively, most of the studies examined only raw materials, which might not give a full picture of quinoa potential and health benefits. Therefore, this paper evaluated the effect of various processing methods and processing time on the CP, TPC, AA (Fig. 3) and the content of selected bioactive compounds (Table 1) of Probio sample. Soaking was proven to be effective for minimizing the content of anti-nutritional compounds [36]. However, our results indicated that soaking in water worsened the majority of studied nutritional parameters. The exceptions were metabolites KMP, NAR, PC, and RUT where soaking led to a rise in their content. Presented alterations after soaking might be related to various factors. The reduction in CP was attributed to a leaching of quinoa seed storage proteins into soaking water [14]. Similarly, a softening of cell-wall tissues could potentially facilitate the increased release of polyphenols into the soaking medium [37], thus possibly reducing the TPC and AA of soaked seeds. In addition, the variability in the metabolite content could be ascribed to the commencement of the seed germination processes, as discussed above.
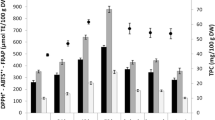
The effect of various processing methods and processing time on crude protein content (CP), antioxidant activity (AA), and total phenolic content (TPC) of commercial Probio sample. Significant differences in means (Tukey HSD) within individual treatments are indicated by different letters (a-c) above each column. The letters (A-E) in the header of plot shows the difference among individual treatments. Dashed red line within each plot denotes overall mean of data for respective variable. The abbreviations for the selected processing methods are as follows: control (C), SK (soaking), B (boiling), B NaCl (boiling in NaCl) (min)
Boiling in plain water and NaCl solution
When comparing two boiling solutions, boiling in plain water showed slightly better results in CP content and the AA, principally after 15 min of treatment, with respect to the boiling in NaCl. The values reported after 15 min of boiling in plain water were relatively similar to the control sample in both parameters. It was previously stated that no significant alterations in CP occurred after 15 min of boiling [6].Nonetheless, the AA could be, in part, influenced by thermally induced modifications in the phenolic content and composition [38].
Overall, boiling in both solutions was evaluated as the least suited method for the TPC enhancement among other examined processing methods. In addition, the TPC was reduced more intensively during the boiling in plain water than in boiling in NaCl solution (Fig. 3). The TPC might be influenced by various factors. Apart from sample variety, processing conditions, and method of analysis, the reasons for the decline in TPC might include the polyphenol leaching into boiling water and their thermal degradation. Conversely, the release of polyphenols and the inactivation of phenol oxidase may contribute to their propensity, as reported previously in buckwheat [37].
This was also reflected in the distinct behaviours of metabolite content during boiling. Boiling in plain water was not suited for the enhancement of 4BA, CFA, IQ, Q3G, and SA (Table 1), but it improved the content of KMP and PC, compared to control. Boiling in NaCl was considered more beneficial in contrast to boiling in plain water, since most of the studied metabolites reached higher mean values in their content. The presence of salt in the solution could possibly increase the boiling point and therefore induce a higher degree of thermal dissociation of bound molecules, as proposed for pulses [39].
Flaking
Flakes from whole quinoa seeds demonstrated a noteworthy reduction in the required boiling time, reducing it to a mere 5 min, with respect to the boiling of whole seeds. Therefore, further utilisation of quinoa flakes could be potentially advantageous in mitigating the heat-induced degradation of thermally unstable compounds. It is noteworthy that research in this specific domain for quinoa remains scarce. The shorter cooking time of quinoa flakes enhanced the TPC and the content of IQ, NAR, and SA, in contrast to the boiled seeds. Moreover, among all the treatments explored in this study (Table 1), boiled flakes exhibited the highest mean content of 4BA and Q3G. In addition, boiled flakes achieved the highest CP values when compared to all the other heat-utilizing methods investigated herein. Nonetheless, the mean AA of boiled flakes was lower than the values of boiled seeds. While the precise impact of boiling of quinoa flakes on the final nutritional quality has not been studied yet, it was previously concluded that flaking of ancient cereals and legumes may increase or decrease the TPC and AA depending on the type of sample. Contrary, the protein content was not significantly affected by flaking [40].
Microwaving
Microwaving was a relatively suitable method for enhancing the protein content since the mean CP of microwaved samples was the second highest among other studied treatments (15.56 ± 0.10%). Furthermore, CP remained unaffected by varying microwave exposure times (Fig. 3). There is a lack of comprehensive studies elucidating the impact of microwaving on quinoa CP, however, studies conducted on other species, such as legumes and buckwheat, indicated quite variable outcomes in this area [41, 42]. While the mean AA values were statistically comparable to roasting and boiling in NaCl, the TPC values for microwaving were outstanding, reaching the peak in the third minute (27.15 ± 0.82 GAE g/kg dw). In a parallel study, a similar reduction in AA with increasing time of processing was noticed, nevertheless, the highest TPC was detected after 5 min of microwaving [43]. Half of the studied metabolites, namely CFA, KMP, NAR, QCE, RUT, and SA, displayed the highest mean content during microwaving (Table 1), in comparison to other heat treatments and raw samples. This observation aligns with the PCA analysis results (Fig. 4), where microwave-treated samples distinctly cluster along the first principal component axis, revealing a strong influence from the mentioned traits. Including all heat treatments, GA was only found in microwaved and roasted samples. As concluded by Drulyte and Orlien [44], the heating effect of microwaving is more intense and faster than alternative cooking methods. This distinctive trait leads to a reduction in overall processing time and, notably, correlates positively with diminished losses of polyphenolic compounds [38]. Our results confirmed the conclusions of other studies that microwaving yields the highest number of polyphenols among other heat treatments, thereby increasing the overall antioxidant capacity [39, 43, 45, 46].
Principal component analysis biplot based on scaled data for set of 14 descriptors and 22 different culinary treatments. Two main components explaining 43.8 and 24.1% of total variance, respectively, are displayed. Individual points in plot stands for individual culinary treatments, highlighted by different colors and variants of those treatments, representing treatments duration in minutes (m.). The arrows within plot shows the quality of representation of individual descriptors on factor map and its contribution to first two axis. The abbreviations for the selected processing methods and descriptors are as follows: control (C), SK (soaking), B (boiling), B NaCl (boiling in NaCl), gallic acid (GA), 4-hydroxybenzaldehyde (4BA), caffeic acid (CFA), quercetin-3-O-glucoronide (Q3G), isoquercetin (IQ), rutin (RUT), salicylic acid (SA), quercetin (QCE), naringenin (NAR), kaempferol (KMP), pinocembrin (PC), isorhamnetin (IH), crude protein content (CP), antioxidant activity (AA), total phenolic content (TPC)
Steaming
Steaming is, together with boiling, a commonly employed methods of quinoa preparation. Even though both processing methods generally led to a reduction in the content of biologically active compounds compared to raw sample [47], steaming is recommended as more optimal method for better nutrient retention over boiling [6]. This preference stems from the fact that, during steaming, the quinoa seeds are not in direct contact with boiling water, thereby minimizing nutrient leaching into the water [8]. Our results confirmed this statement since the TPC in steamed sample was higher than in boiled samples. In addition, steaming did not affect protein content in quinoa seeds, aligning with previously published research [6, 48] although in contrast with Motta et al. [49], who reported a significant decrease in CP in studied pseudocereals (Amaranthus sp., quinoa and buckwheat). In terms of studied metabolites, their quantity was either comparable or lower than those observed in other heat treatments, except for IH, reaching the highest value in this study (1.60 ± 0.04 μg/g dw) after 15 min of steaming (Table 1).
Roasting
Roasted quinoa seed did not reach any outstanding values for the content of protein and AA since both parameters were statistically comparable to boiling in plain water and boiling in NaCl, respectively (Fig. 3). Nevertheless, roasted seeds exhibited a great content of total polyphenols, reaching values comparable to the control after 15 min of roasting. The overall increment in TPC during roasting can be attributed to the release of bound chemicals due to heat and the formation of Maillard reaction products, but the yield of phenolics is also influenced by the roasting temperature and time used during processing [11]. This might explain the contradicting results of some studies, showing the TPC and AA of roasted seed with values even higher than control sample [43, 46] and others with significantly reduced polyphenolic content [8]. In case of metabolite content, roasting was a suitable technique for the enhancement of IQ, KMP, SA, RUT, and QCE, especially after 15 min of roasting time (Table 1). Similar metabolites were investigated previously in amaranth [50], nonetheless, the pattern of the changes during roasting was distinct from our results. For example, QCE and KMP significantly decreased after 15 min of roasting, whereas GA and CFA increased rapidly.
Conclusion
The present investigation was conducted to assess the impact of germination, soaking, boiling, flaking, microwaving, steaming, and roasting on the selected nutritional characteristics of quinoa. The quantitative analysis of 12 bioactive compounds was conducted in three distinct quinoa samples during a 5-day germination period. In all studied samples, GA, CFA, RUT, QCE, NAR, KMP, IH, and PC were enhanced compared to control, but the level of increment was contingent upon the type of sample. This underscores the importance of proper selection of genotype for optimum content of biologically active compounds in germinated quinoa. The most substantial increase in bioactive compounds was noticed between the third and fifth day of germination with the highest accumulation of metabolites occurring in the genotypes ‘Besançon’ and ‘Faro’. Six compounds (NAR, SA, 4BA, IQ, PC, IH) were detected in germinated quinoa for the first time.
This study further examined a range of various heat-utilizing methods. Statistically significant differences were observed in CP among heat treatments. Boiled quinoa flakes exhibited the highest average protein content and proved to be a time-efficient preparation method due to reduced boiling time. The lowest mean values of CP were associated with roasting and boiling. Most of the heat treatments caused a decrease in TPC and AA in comparison to raw sample. An exception to this trend was microwaving which strongly enhanced the overall TPC of quinoa sample and the content of several metabolites (CFA, KMP, NAR, QCE, RUT, and SA).
It can be concluded that different processing methods influenced the nutritional content and composition of quinoa differently. The specific effects varied depending on the processing technique, duration of treatment, compound measured, and the genotype. Nonetheless, further research is warranted to elucidate the underlying mechanisms driving these changes. The alterations observed in this study emphasize the importance of considering those variables in optimizing the processing methods used for quinoa to obtain the best nutritional profile of final food product. Therefore, this knowledge contributes to the development of processing techniques that preserve or enhance the nutritional value of quinoa and promote its utilization as a source of health-promoting compounds in human diets.
Data availability
All of the data have been listened in manuscript and Supplementary material.
Abbreviations
- ANOVA:
-
A two-way analysis of variance
- AA:
-
Antioxidant activity
- 4BA:
-
4-Hydroxybenzaldehyde
- FA:
-
Caffeic acid
- FC:
-
Folin–Ciocalteau
- CP:
-
Crude protein
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ESI:
-
Electrospray ionization
- GA:
-
Gallic acid
- G1D:
-
1 Day of germination
- G2D:
-
2 Day of germination
- G3D:
-
3 Days of germination
- G4D:
-
4 Days of germination
- G5D:
-
5 Days of germination
- IH:
-
Isorhamnetin
- IQ:
-
Isoquercetin
- KMP:
-
Kaempferol
- NAR:
-
Naringenin
- PC:
-
Pinocembrin
- TE:
-
Trolox equivalent
- QCE:
-
Quercetin
- Q3G:
-
Quercetin 3-O-glucuronide
- RUT:
-
Rutin
- SA:
-
Salicylic acid
- TFC:
-
Total flavonoid content
- TPC:
-
Total phenolic content
References
Jacobsen SE. Mujica A (2002) Genetic resources and breeding of the Andean grain crop quinoa (Chenopodium quinoa Willd.), IPGRI: Rome, Italy, pp 54–61
Alandia G, Rodriguez JP, Jacobsen SE, Bazile D, Condori B (2020) Global expansion of quinoa and challenges for the Andean region. Glob Food Secur Agric Policy 26:10. https://doi.org/10.1016/j.gfs.2020.100429
Abderrahim F, Huanatico E, Segura R, Arribas S, Gonzalez MC, Condezo-Hoyos L (2015) Physical features, phenolic compounds, betalains and total antioxidant capacity of coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chem 183:83–90. https://doi.org/10.1016/j.foodchem.2015.03.029
Vakevainen K, Ludena-Urquizo F, Korkala E, Lapvetelainen A, Peraniemi S, von Wright A, Plumed-Ferrer C (2020) Potential of quinoa in the development of fermented spoonable vegan products. Lwt-Food Sci Technol 120:108912. https://doi.org/10.1016/j.lwt.2019.108912
Balakrishnan G, Schneider RG (2022) The role of amaranth, quinoa, and millets for the development of healthy, sustainable food products-a concise review. Foods 11(16):2442. https://doi.org/10.3390/foods11162442
Mhada M, Metougui ML, El Hazzam K, El Kacimi K, Yasri A (2020) Variations of saponins, minerals and total phenolic compounds due to processing and cooking of quinoa (Chenopodium quinoa Willd.) seeds. Foods 9(5):660. https://doi.org/10.3390/foods9050660
Bhinder S, Kumari S, Singh B, Kaur A, Singh N (2021) Impact of germination on phenolic composition, antioxidant properties, antinutritional factors, mineral content and Maillard reaction products of malted quinoa flour. Food Chem 346:128915
Nickel J, Spanier LP, Botelho FT, Gularte MA, Helbig E (2016) Effect of different types of processing on the total phenolic compound content, antioxidant capacity, and saponin content of Chenopodium quinoa Willd grains. Food Chem 209:139–143. https://doi.org/10.1016/j.foodchem.2020.128915
Lorusso A, Verni M, Montemurro M, Coda R, Gobbetti M, Rizzello CG (2017) Use of fermented quinoa flour for pasta making and evaluation of the technological and nutritional features. Lwt-Food Sci Technol 78:215–221. https://doi.org/10.1016/j.lwt.2016.12.046
Cao HW, Huang QL, Shi JR, Guan X, Song HD, Zhang Y, Xie J, Fang Y (2023) Effect of conventional and microwave heating treatment on antioxidant activity of quinoa protein after simulated gastrointestinal digestion. Food Chem 415:135763. https://doi.org/10.1016/j.foodchem.2023.135763
Carciochi RA, D’Alessandro LG, Manrique GD (2016) Effect of roasting conditions on the antioxidant compounds of quinoa seeds. Int J Food Sci Technol 51(4):1018–1025
Demir B, Bilgicli N (2020) Changes in chemical and anti-nutritional properties of pasta enriched with raw and germinated quinoa (Chenopodium quinoa Willd.) flours. J Food Sci Tech 57(10):3884–3892. https://doi.org/10.1007/s13197-020-04420-7
Thakur P, Kumar K, Ahmed N, Chauhan D, Rizvi QUH, Jan S, Singh TP, Dhaliwal HS (2021) Effect of soaking and germination treatments on nutritional, anti-nutritional, and bioactive properties of amaranth (Amaranthus hypochondriacus L.), quinoa (Chenopodium quinoa L.), and buckwheat (Fagopyrum esculentum L.). Curr Res Food Sci 4:917–925. https://doi.org/10.1016/j.crfs.2021.11.019.eCollection2021
Maldonado-Alvarado P, Pavon-Vargas DJ, Abarca-Robles J, Valencia-Chamorro S, Haros CM (2023) Effect of germination on the nutritional properties, phytic acid content, and phytase activity of Quinoa (Chenopodium quinoa Willd). Foods 12(2):389. https://doi.org/10.3390/foods12020389
Jimenez MD, Lobo M, Samman N (2019) 12th IFDC 2017 Special Issue—influence of germination of quinoa (Chenopodium quinoa) and amaranth (Amaranthus) grains on nutritional and techno-functional properties of their flours. J Food Compost Anal 84:103290. https://doi.org/10.1016/j.jfca.2019.103290
Pilco-Quesada S, Tian Y, Yang BR, Repo-Carrasco-Valencia R, Suomela JP (2020) Effects of germination and kilning on the phenolic compounds and nutritional properties of quinoa (Chenopodium quinoa) and kiwicha (Amaranthus caudatus). J Cereal Sci 94:102996. https://doi.org/10.1016/j.jcs.2020.102996
Janovska D, Jagr M, Svoboda P, Dvoracek V, Meglic V, Hlasna Cepkova P (2021) Breeding buckwheat for nutritional quality in the Czech Republic. Plants 10(7):1262. https://doi.org/10.3390/plants10071262
Czech State Norm 5983-1 (461014). Obiloviny a luštěniny—Stanoveníobsahudusíku a výpočetobsahudusíkatýchlátek—Kjeldahlovametoda (in Czech). 2014; p 24
Holasova M, Fiedlerova V, Smrcinova H, Orsak M, Lachman J, Vavreinova S (2002) Buckwheat—the source of antioxidant activity in functional foods. Food Res Int 35(2–3):207–211. https://doi.org/10.1016/s0963-9969(01)00185-5
Sensoy I, Rosen RT, Ho CT, Karwe MV (2006) Effect of processing on buckwheat phenolics and antioxidant activity. Food Chem 99(2):388–393. https://doi.org/10.1016/j.foodchem.2005.08.007
R Core Team (2020) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Lê S, Josse J, Husson F (2008) FactoMineR: An R Package for Multivariate Analysis. J Stat Soft 25(1):1–18. https://doi.org/10.18637/jss.v025.i01
Kassambara A, Mundt F (2020) Factoextra: extract and visualize the results of multivariate data analyses. R Package Version 1:7
Hejazi SN, Orsat V (2016) Malting process optimization for protein digestibility enhancement in finger millet grain. J Food Sci Technol 53(4):1929–1938. https://doi.org/10.1007/s13197-016-2188-x
Guardianelli LM, Salinas MV, Brites C, Puppo MC (2022) Germination of white and red quinoa seeds: improvement of nutritional and functional quality of flours. Foods 11(20):3272. https://doi.org/10.3390/foods11203272
Xu MW, Rao JJ, Chen BC (2020) Phenolic compounds in germinated cereal and pulse seeds: classification, transformation, and metabolic process. Crit Rev Food Sci Nutr 60(5):740–759. https://doi.org/10.1080/10408398.2018.1550051
Paucar-Menacho LM, Martínez-Villaluenga Ch, Dueňas M, Frias J, Peňas E (2017) Response surface optimisation of germination conditions to improve the accumulation of bioactive compounds and the antioxidant activity in quinoa. Int J Food Sci Technol 53:516–524. https://doi.org/10.1111/ijfs.13623
Al-Qabba MM, El-Mowafy MA, Althwab SA, Alfheeaid HA, Aljutaily T, Barakat H (2020) Phenolic profile, antioxidant activity, and ameliorating efficacy of Chenopodium quinoa sprouts against CCl4-induced oxidative stress in rats. Nutrients 12(10):2904. https://doi.org/10.3390/nu12102904
Gan RY, Lui WY, Wu K, Chan CL, Dai SH, Sui ZQ, Corke H (2017) Bioactive compounds and bioactivities of germinated edible seeds and sprouts: an updated review. Trends Food Sci Technol 59:1–14. https://doi.org/10.1016/j.tifs.2016.11.010
Ahmed S, Al-Rehaily AJ, Alam P, Alqahtani AS, Hidayatullah S, Rehman MT, Mothana RA, Abbas SS, Khan MU, Khalid JM, Siddiqui NA (2019) Antidiabetic, antioxidant, molecular docking and HPTLC analysis of miquelianin isolated from Euphorbia schimperi C. Presl. Saudi Pharm J 27(5):655–663. https://doi.org/10.1016/j.jsps.2019.03.008
Kashchenko NI, Olennikov DN, Chirikova NK (2021) Metabolites of Siberian raspberries: LC-MS profile, seasonal variation, antioxidant activity and thermal stability of Rubus matsumuranus phenolome. Plants 10(11):2317. https://doi.org/10.3390/plants10112317
Wang Z, Xue C, Yang T, Zeng M, Wang Z, Chen Q, Chen J, He Z (2023) Miquelianin, amain functional flavonoid of lotus leaf, induces thermogenic signature via p38-PINK1-PARKIN-mediated mitophagy andmimicking NRF2 signaling during brown adipocyte differentiation. Food Front 2023:1–14. https://doi.org/10.1002/fft2.284
Tang DY, Dong YM, Guo N, Li L, Ren HK (2013) Metabolomic analysis of the polyphenols in germinating mung beans (Vigna radiata) seeds and sprouts. J Sci Food Agric 94(8):1639–1647. https://doi.org/10.1002/jsfa.6471
Dumitru C, Dinica RM, Bahrim GE, Vizireanu C, Baroiu L, Iancu AV, Draganescu M (2021) New insights into the antioxidant compounds of achenes and sprouted buckwheat cultivated in the republic of Moldova. Appl Sci 11(21):10230. https://doi.org/10.3390/app112110230
Ng CY, Wang MF (2021) The functional ingredients of quinoa (Chenopodium quinoa) and physiological effects of consuming quinoa: a review. Food Front 2(3):329–356. https://doi.org/10.1002/fft2.109
Lalaleo L, Hidalgo D, Valle M, Calero-Caceres W, Lamuela-Raventos RM, Becerra-Martinez E (2020) Differentiating, evaluating, and classifying three quinoa ecotypes by washing, cooking and germination treatments, using H-1 NMR-based metabolomic approach. Food Chem 331:127351. https://doi.org/10.1016/j.foodchem.2020.127351
Yang QH, Luo Y, Wang HL, Li J, Gao XL, Gao JF, Feng BL (2021) Effects of germination on the physicochemical, nutritional and in vitro digestion characteristics of flours from waxy and nonwaxy proso millet, common buckwheat and pea. Innov Food Sci Emerg Technol 67(21):102586. https://doi.org/10.1016/j.ifset.2020.102586
Murador D, Braga AR, Da Cunha D, De Rosso V (2018) Alterations in phenolic compound levels and antioxidant activity in response to cooking technique effects: a meta-analytic investigation. Crit Rev Food Sci Nutr 58(2):169–177. https://doi.org/10.1080/10408398.2016.1140121
Liu YH, Ragaee S, Marcone MF, Abdel-Aal EM (2020) Composition of phenolic acids and antioxidant properties of selected pulses cooked with different heating conditions. Foods 9(7):908. https://doi.org/10.3390/foods9070908
Consumi M, Tamasi G, Bonechi C, Andreassi M, Leone G, Magnani A, Rossi C (2022) Effect of flaking and precooking procedures on antioxidant potential of selected ancient cereal and legume flours. Foods 11(11):1592. https://doi.org/10.3390/foods11111592
Uppal V, Bains K (2012) Effect of germination periods and hydrothermal treatments on in vitro protein and starch digestibility of germinated legumes. J Food Sci Technol 49(2):184–191. https://doi.org/10.1007/s13197-011-0273-8
Sharma S, Kataria A, Singh B (2022) Effect of thermal processing on the bioactive compounds, antioxidative, antinutritional and functional characteristics of quinoa (Chenopodium quinoa). Lwt-Food Sci Technol 160:113256. https://doi.org/10.1016/j.lwt.2022.113256
Deng Y, Padilla-Zakour O, Zhao YY, Tao SS (2015) Influences of high hydrostatic pressure, microwave heating, and boiling on chemical compositions, antinutritional factors, fatty acids, in vitro protein digestibility, and microstructure of buckwheat. Food Bioproc Tech 8(11):2235–2245. https://doi.org/10.1007/s11947-015-1578-9
Drulyte D, Orlien V (2019) The effect of processing on digestion of legume proteins. Foods 8(6):224. https://doi.org/10.3390/foods8060224
Kheto A, Joseph D, Islam M, Dhua S, Das R, Kumar Y, Vashishth R, Sharanagat VS, Kumar K, Nema PK (2022) Microwave roasting induced structural, morphological, antioxidant, and functional attributes of Quinoa (Chenopodium quinoa Willd). J Food Process Preserv 46(5):e16595. https://doi.org/10.1111/jfpp.16595
Zhang Y, Yan Y, Li WQ, Huang K, Li S, Cao HW, Guan X (2022) Microwaving released more polyphenols from black quinoa grains with hypoglycemic effects compared with traditional cooking methods. J Sci Food Agric 102(13):5948–5956. https://doi.org/10.1002/jsfa.11947
Gu RJ, Chang XW, Bai GT, Li X, Di Y, Liu XB, Sun LJ, Wang YT (2021) Effects of household cooking methods on changes of tissue structure, phenolic antioxidant capacity and active component bioaccessibility of quinoa. Food Chem 350:129138. https://doi.org/10.1016/j.foodchem.2021.129138
Wang L, Dong J-l, Zhu Y-y, Shen R-l, Wu L-g, Zhang K-y (2021) Effects of microwave heating, steaming, boiling and baking on the structure and functional properties of quinoa (Chenopodium quinoa Willd.) protein isolates. Int J Food Sci Technol 56:709–720. https://doi.org/10.1111/ijfs.14706
Motta C, Castanheira I, Gonzales GB, Delgado I, Torres D, Santos M, Matos AS (2019) Impact of cooking methods and malting on amino acids content in amaranth, buckwheat and quinoa. J Food Compost Anal 76:58–65. https://doi.org/10.1016/j.jfca.2018.10.001
Juhaimi FA, Ahmed IAM, Özcan MM, Uslu N (2023) Effect of roasting times on bioactive compounds, fatty acids, polyphenol and nutrients of amaranth (Amaranthus cruentus L.) seed roasted in pan, and principal component analysis. J Food Sci Technol. https://doi.org/10.1007/s13197-023-05828-7
Funding
Open access publishing supported by the National Technical Library in Prague. This work was financially supported by the Ministry of Agriculture of the Czech Republic, institutional support MZE-RO0423 and co-funded by the Internal Grant Agency of the Faculty of Tropical AgriSciences, Czech University of Life Sciences Prague, IGA (Project No. 20233115). We gratefully acknowledge the National Programme for the Conservation and Use of Plant Genetic Resources and Agrobiodiversity (no. MZE-62216/2022-13113/6.2.14) and PRO-BIO Ltd, Czech Republic for providing plant material.
Author information
Authors and Affiliations
Contributions
Conceptualization: PHČ; Methodology: PHČ, MJ, LD; Formal analysis and investigation: LD, MJ, PHČ; Data curation: PS; Visualization: PS; Writing—original draft preparation: LD; Writing—review and editing: PHČ, IV; Funding acquisition: DJ, VD; Resources: DJ; Supervision: PHČ, IV.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dostalíková, L., Hlásná Čepková, P., Janovská, D. et al. The impact of germination and thermal treatments on bioactive compounds of quinoa (Chenopodium quinoa Willd.) seeds. Eur Food Res Technol 250, 1457–1471 (2024). https://doi.org/10.1007/s00217-024-04466-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-024-04466-3