Abstract
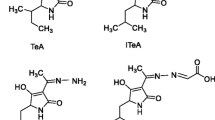
Tenuazonic acid (TeA), as a naturally occurring mycotoxin found in sorghum, corn, wheat, oat, tomato, apple, rapeseed and their products, has attracted increasing attention due to its wide contamination and high dietary exposure of infants and toddlers. Traditional methods usually require large instruments and high working conditions but are not suitable for low-cost large-scale screening. Thus, a rapid, sensitive and inexpensive method for TeA detection is required. Using the highly specific TeA monoclonal antibody (McAb), a novel indirect competitive chemiluminiscence enzyme immunoassay (icCLEIA) of tenuazonic acid (TeA) detection was developed. Based on the chemiluminescence system of bis(2,4,6-trichlorophenyl)oxalate-urea peroxide-3-(4-hydroxyphenyl)propanoic acid (TCPO–CH4N2O·H2O2–PHPPA) dimer, the enhancement of luminescence intensity for TeA determination was achieved. Under the optimal conditions, this method displayed a good linear range (0.06–1.62 ng mL−1) with lower IC50 (0.16 ng mL−1) and LOD (0.01 ng mL−1/7.5 μg kg−1) compared with other assays. Besides, the recoveries of spiked wheat flour and oat samples tested were 76.24–88.95% and 85.34–93.28%, respectively. The results manifested that the newly developed method with the advantages of fast and high sensitivity can be used for the trace detection of TeA in foodstuffs.






Similar content being viewed by others
References
Puntscher H, Kuett ML, Skrinjar P, Mikula H, Podlech J, Frohlich J, Marko D, Warth B (2018) Tracking emerging mycotoxins in food: development of an LC-MS/MS method for free and modified Alternaria toxins. Anal Bioanal Chem 410(18):4481–4494
Aichinger G, Krueger F, Puntscher H, Preindl K, Warth B, Marko D (2019) Naturally occurring mixtures of Alternaria toxins: anti-estrogenic and genotoxic effects in vitro. Arch Toxicol 93(10):3021–3031
Escrivá L, Oueslati S, Font G, Manyes L (2017) Alternaria mycotoxins in food and feed: an overview. J Food Qual:1569748
Fraeyman S, Croubels S, Devreese M, Antonissen G (2017) Emerging Fusarium and Alternaria mycotoxins: occurrence, toxicity and toxicokinetics. Toxins 9(7):228
Lehmann L, Wagner J, Metzler M (2006) Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells. Food Chem Toxicol 44(3):398–408
Bernal ARR, Reynoso CM, Londono VAG, Broggi LE, Resnik SL (2019) Alternaria toxins in Argentinean wheat, bran, and flour. Food Addit Contam B 12(1):24–30
Zwickel T, Klaffke H, Richards K, Rychlik M (2016) Development of a high performance liquid chromatography tandem mass spectrometry based analysis for the simultaneous quantification of various Alternaria toxins in wine, vegetable juices and fruit juices. J Chromatogr A 1455:74–85
Masiello M, Somma S, Susca A, Ghionna V, Logrieco AF, Franzoni M, Ravaglia S, Meca G, Moretti A (2020) Molecular identification and mycotoxin production by Alternaria species occurring on durum wheat, showing black point symptoms. Toxins 12(4):275
Rychlik M, Lepper H, Weidner C, Asam S (2016) Risk evaluation of the Alternaria mycotoxin tenuazonic acid in foods for adults and infants and subsequent risk management. Food Control 68:181–185
de Oliveira RC, Carnielli-Queiroz L, Correa B (2018) Epicoccum sorghinum in food: occurrence, genetic aspects and tenuazonic acid production. Curr Opin Food Sci 23:44–48
Sanzani SM, Gallone T, Garganese F, Caruso AG, Amenduni M, Ippolito A (2019) Contamination of fresh and dried tomato by Alternaria toxins in southern Italy. Food Addit Contam A 36(5):789–799
Mujahid C, Savoy MC, Basle Q, Woo PM, Ee ECY, Mottier P, Bessaire T (2020) Levels of alternaria toxins in selected food commodities including green coffee. Toxins 12(9):595
Ostry V (2008) Alternaria mycotoxins: an overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J 1(2):175–188
Hessel-Pras S, Kieshauer J, Roenn G, Luckert C, Braeuning A, Lampen A (2019) In vitro characterization of hepatic toxicity of Alternaria toxins. Mycotoxin Res 35(2):157–168
EFSA (2016) Dietary exposure assessment to Alternaria toxins in the European population. EFSA J 14(12):4654. https://www.efsa.europa.eu/en/efsajournal/pub/4654
EFSA (2011) Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J 9(10):2407. https://doi.org/10.2903/j.efsa.2011.2407
Hickert S, Bergmann M, Ersen S, Cramer B, Humpf HU (2016) Survey of Alternaria toxin contamination in food from the German market, using a rapid HPLC-MS/MS approach. Mycotoxin Res 32(1):7–18
Prelle A, Spadaro D, Garibaldi A, Gullino ML (2013) A new method for detection of five Alternaria toxins in food matrices based on LC-APCI-MS. Food Chem 140(1–2):161–167
Yao CY, Xu ZL, Wang H, Zhu F, Luo L, Yang JY, Sun YM, Lei HT, Tian YX, Shen YD (2019) High affinity antibody based on a rationally designed hapten and development of a chemiluminescence enzyme immunoassay for quantification of alternariol in fruit juice, maize and flour. Food Chem 283:359–366
Zhao FC, Shi RR, Liu RX, Tian Y, Yang ZY (2021) Application of phage-display developed antibody and antigen substitutes in immunoassays for small molecule contaminants analysis: a mini-review. Food Chem 339:128084
Gao HF, Han J, Yang SJ, Wang ZX, Wang L, Fu ZF (2014) Highly sensitive multianalyte immunochromatographic test strip for rapid chemiluminescent detection of ractopamine and salbutamol. Anal Chim Acta 839:91–96
Xu L, Zhang Z, Zhan Q, Li PW (2016) Mycotoxin determination in foods using advanced sensors based on antibodies or aptamers. Toxins 8(8):239
Roda A, Manetta AC, Piazza F, Simoni P, Lelli R (2000) A rapid and sensitive 384-microtiter wells format chemiluminescent enzyme immunoassay for clenbuterol. Talanta 52(2):311–318
You XD, Arrowood MJ, Lejkowski M, Xie LT, Schinazi RF, Mead JR (1996) A chemiluminescence immunoassay for evaluation of Cryptosporidium parvum growth in vitro. Fems Microbiol Lett 136(3):251–256
Chandross EA (1963) A new chemiluminescent system. Tetrahedron Lett 12:761–765
Grayeski ML, Seitz WR (1984) Determination of fluorophor-labeled compounds based on peroxyoxalate chemiluminescence. Anal Biochem 136(2):277–284
Capomacchia AC, Jennings RN, Hemingway SM, Dsouza P, Prapaitrakul W, Gingle A (1987) Native peroxyoxalate chemiluminescence from the reaction of bis(2,4-dinitrophenyl)oxalate and hydrogen peroxide perturbed by nonfluorophores. Anal Chim Acta 196:305–310
Rameil S, Schubert P, Grundmann P, Dietrich R, Martlbauer E (2010) Use of 3-(4-hydroxyphenyl)propionic acid as electron donating compound in a potentiometric aflatoxin M1-immunosensor. Anal Chim Acta 661(1):122–127
Zhong H (2017) Study on rapid and high sensitive quantitative detection kit for tenuazonic acid and preparation of anti-tenuazonic acid monoclonal antibody [D]. Southwest University, Chongqing
Wang C, Fan YY, He WZ, Hu DQ, Wu AB, Wu WL (2018) Development and application of a QuEChERS-based liquid chromatography tandem mass spectrometry method to quantitate multi-component Alternaria toxins in jujube. Toxins 10(10):382
Wu Y, Ye J, Xuan ZH, Li L, Wang HB, Wang SS, Liu HM, Wang SX (2021) Development and validation of a rapid and efficient method for simultaneous determination of mycotoxins in coix seed using one-step extraction and UHPLC-HRMS. Food Addit Contam A 38(1):148–159
Gross M, Asam S, Rychlik M (2017) Evaluation of an enzyme immunoassay for the detection of the mycotoxin tenuazonic acid in sorghum grains and sorghum-based infant food. Mycotoxin Res 33(1):75–78
Wang F, Li ZF, Yang YY, Wan DB, Vasylieva N, Zhang YQ, Cai J, Wang H, Shen YD, Xu ZL, Hammock BD (2020) Chemiluminescent enzyme immunoassay and bioluminescent enzyme immunoassay for tenuazonic acid mycotoxin by exploitation of nanobody and nanobody-nanoluciferase fusion. Anal Chem 92(17):11935–11942
Liang YF, Zhou XW, Wang F, Shen YD, Xiao ZL, Zhang SW, Li YJ, Wang H (2020) Development of a monoclonal antibody-based ELISA for the detection of alternaria mycotoxin tenuazonic acid in food samples. Food Anal Methods 13(8):1594–1602
Wang F, Wan DB, Shen YD, Tian YX, Xiao ZL, Xu ZL, Yang JY, Sun YM, Hammock BD, Wang H (2021) Development of a chemiluminescence immunoassay for detection of tenuazonic acid mycotoxin in fruit juices with a specific camel polyclonal antibody. Anal Methods 13(15):1795–1802
Wang F, Li ZF, Wan DB, Vasyliesa N, Shen YD, Xu ZL, Yang JY, Gettemans J, Wang H, Hammock BD, Sun YM (2021) Enhanced non-toxic immunodetection of Alternaria mycotoxin tenuazonic acid based on ferritin-displayed anti-idiotypic nanobody-nanoluciferase multimers. J Agr Food Chem 69(16):4911–4917
Yang XX, Liu XX, Wang H, Xu ZL, Shen YD, Sun YM (2012) Development of an enzyme-linked immunosorbent assay method for the detection of tenuazonic acid. Chinese J Anal Chem 40(9):1347–1352 (in Chinese)
Liang Y, Wang Y, Wang F, Li J, Wang C, Dong J, Ueda H, Xiao Z, Shen Y, Xu Z, Wang H (2021) An enhanced open sandwich immunoassay by molecular evolution for noncompetitive detection of Alternaria mycotoxin tenuazonic acid. Food Chem 361:130103
Acknowledgements
This study was supported by the National Natural Science Foundation of China [No. 32001810], the Chongqing Research Program of Basic Research and Frontier Technology [No. cstc2019jcyj-msxmX0278], and the Venture and Innovation Support Program for Chongqing Overseas Returnees [No. cx2018032]. We are very grateful to the people who gave us any advice and help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Compliance with ethics requirements
All experiments conformed to the established guidelines for the Laboratory Animal Welfare and Ethics Committee of Southwest University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, H., Pan, S., Tan, H. et al. A novel high-sensitive indirect competitive chemiluminescence enzyme immunoassay based on monoclonal antibody for tenuazonic acid (TeA) detection. Eur Food Res Technol 248, 577–587 (2022). https://doi.org/10.1007/s00217-021-03905-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03905-9