Abstract
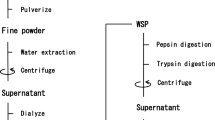
Angiotensin I converting enzyme (ACE) inhibition is one of the key factors for cardiovascular disease, and inhibition of ACE activity is related to the prevention of high blood pressure. Until now, some ACE inhibitory peptides have been found in the protein hydrolysates of red algae, and most of them were derived from phycobiliproteins and rubisco. In this study, we modified the preparation method to evaluate the potential of ACE inhibitory activity from protein hydrolysate of red alga Mazzaella japonica except for phycobiliproteins and rubisco. As a result, we identified 11 peptides (YRD, VSEGLD, TIMPHPR, GGPAT, SSNDYPI, SRIYNVKSNG, VDAHY, CPYDWV, YGDPDHY, NLGN and DFGVPGHEP) in the hydrolysate by the reversed phase-HPLC and MALDI-TOF/MS/MS. Among them, YRD would be derived from phycoerythrin α-subunit. However, the others would be from other proteins encoded in chloroplast or nuclear genomes. This study revealed that not only phycobiliproteins and rubisco but also the other proteins in red algae have the potential for the source of ACE inhibitory peptide.

Similar content being viewed by others
References
World Health Organization. https://www.who.int
Tsai JS, Lin TC, Chen JL, Pan BS (2017) The inhibitory effects of freshwater clam (Corbicula fluminea, Muller) muscle protein hydrolysates on angiotensin I converting enzyme. Process Biochem 41:2276–2281
Chen J, Wang Y, Ye R, Wu Y, Xia W (2013) Comparison of analytical methods to assay inhibitors of angiotensin I-converting enzyme. Food Chem 141:3329–3334
Sweitzer NK (2003) What is an angiotensin converting enzyme inhibitor? Circulation 108:E16–E18
Tu M, Cheng S, Lu W, Du M (2018) Advancement and prospects of bioinformatics analysis for studying bioactive peptides from food-derived protein: Sequence, structure, and functions. Trac-Trend Anal Chem 105:7–17
Li G-H, Le G-W, Shi Y-H, Shrestha S (2004) Angiotensin I–converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr Res 24:469–486
Balti R, Bougatef A, Sila A, Guillochon D, Dhulster P, Nedjar-Arroume N (2015) Nine novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats. Food Chem 170:519–525
García-Moreno PJ, Espejo-Carpio FJ, Guadix A, Guadix EM (2015) Production and identification of angiotensin I-converting enzyme (ACE) inhibitory peptides from Mediterranean fish discards. J Funct Foods 18:95–105
Amado IR, Vázquez JA, González P, Esteban-Fernández D, Carrera M, Piñeiro C (2014) Identification of the major ACE-inhibitory peptides produced by enzymatic hydrolysis of a protein concentrate from cuttlefish wastewater. Mar Drugs 12:1390–1405
Ghassem M, Babji AS, Said M, Mahmoodani F, Aeihara K (2014) Angiotensin I-converting enzyme inhibitory peptides from snakehead fish sarcoplasmic protein hydrolysate. J Food Biochem 38:140–149
Harnedy P-A, FitzGerald R-J (2011) Bioactive proteins, peptides, and amino acid from macroalgae. J Appl Phycol 47:218–232
Sato M, Hosokawa T, Yamaguchi T, Nakano T, Muramoto K, Kahara T, Funayama K, Kobayashi A, Nakano T (2002) Angiotensin I-converting enzyme inhibitory peptides derived from wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J Agric Food Chem 50:6245–6252
Suetsuna K, Maekawa K, Chen J-R (2004) Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J Nutr Biochem 15:267–272
Cha S-H, Lee K-W, Jeon Y-J (2006) Screening of extracts from red algae in Jeju for potentials marine angiotensin-I converting enzyme (ACE) inhibitory activity. Algae 21:343–348
He H-L, Chen X-L, Wu H, Sun C-Y, Zhang Y-Z, Zhou B-C (2007) High throughput and rapid screening of marine protein hydrolysates enriched in peptides with angiotensin-I-converting enzyme inhibitory activity by capillary electrophoresis. Bioresour Thechnol 98:3499–3505
Cian RE, Alaiz M, Vioque J, Drago SR (2013) Enzyme proteolysis enhanced extraction of ACE inhibitory and antioxidant compounds (peptides and polyphenols) from Porphyra columbina residual cake. J Appl Phycol 25:1197–1206
Fitzgerald C, Aluko RE, Hossain M et al (2014) Potential of a renin inhibitory peptide from the red seaweed Palmaria palmata as a functional food ingredient following confirmation and characterization of a hypotensive effect in spontaneously hypertensive rats. J Agric Food Chem 62:8352–8356
Cian R, Garzón A-G, Ancona D-B, Guerrero L-C, Drago S (2015) Hydrolyzates from Pyropia columbina seaweed have antiplatelet aggregation, antioxidant and ACE I inhibitory peptides which maintain bioactivity after simulated gastrointestinal digestion. LWT-Food Sci Technol 64:881–888
Cao D, Lv X, Xu X, Yu H, Sun X, Xu N (2017) Purification and identification of a novel ACE inhibitory peptide from marine alga Gracilariopsis lemaneiformis protein hydrolysate. Eur Food Res Technol 243:1829–1837
Admassu H, Gasmalla MAA, Yang R, Zhao W (2018) Identification of bioactive peptides with α-amylase inhibitory potential from enzymatic protein hydrolysates of red seaweed (Porphyra spp). J Agric Food Chem 66:4872–4882
Cermeño M, Stack J, Tobin P-R, O'Keeffe M-B, Harnedy P-A, Stengel D-B, FitzGerald R-J (2019) Peptide identification from a Porphyra dioica protein hydrolysate with antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. Food Funct 10:3421–3429
Furuta T, Miyabe Y, Yasui H, Kinoshita Y, Kishimura H (2016) Angiotensin I converting enzyme inhibitory peptides derived from phycobiliproteins of dulse Palmaria palmata. Mar Drugs 14:E32
Sumikawa K, Takei K, Kumagai Y, Shimizu T, Yasui H, Kishimura H (2020) In Silico analysis of ACE inhibitory peptides from chloroplast proteins of red alga Grateloupia asiatica. Mar Biotechnol 246:323–332
Kumagai Y, Miyabe Y, Takeda T, Adachi K, Yasui H, Kishimura H (2019) In silico analysis of relationship between proteins from plastid genome of red alga Palmaria sp. (Japan) and angiotensin I converting enzyme inhibitory peptides. Mar Drugs 17:E190
Kumagai Y, Tsubouchi R, Miyabe Y, Takeda T, Adachi K, Yasui H, Kishimura H (2019) Complete sequence of mitochondrial DNA of red alga dulse Palmaria palmata (Linnaeus) Weber & Mohr in Japan. Mitochondrial DNA B 4:3177–3178
Kitade Y, Miyabe Y, Yamamoto Y, Takeda H, Shimizu T, Yasui H, Kishimura H (2017) Structural characteristics of phycobiliproteins from red alga Mazzaella japonica. J Food Biochem 42:E12436
Baldwin J-E, Adlington R-M, Russell A-T, Smith M-L (1995) Carbon based nucleophilic ring opening of activated monocyclic β-lactams; synthesis and stereochemical assignment of the ACE inhibitor WF-10129. Tetrahedron 51:4733–4762
Hooper N-M (1991) Angiotensin converting enzyme: implications from molecular biology for its physiological functions. Int J Biochem 23:641–647
Kobayashi M, Kumagai Y, Yamamoto Y, Yasui H, Kishimura H (2020) Identification of a key enzyme for the hydrolysis of β-(1→3)-xylosyl linkage in red alga dulse xylooligosaccharide from Bifidobacterium adolescentis. Mar Drugs 18:E174
Yamamoto Y, Kishimura H, Kinoshita Y, Saburi W, Kumagai Y, Yasui H, Ojima T (2019) Enzymatic production of xylooligosaccharides from red alga dulse (Palmaria sp.) wasted in Japan. Process Biochem 82:117–122
Leblanc C, Boyen C, Richard O et al (1995) Complete sequence of the mitochondrial DNA of the rhodophyte Chondrus crispus (Gigartinales). Gene content and genome organization. J Mol Biol 250:484–495
Moreira D, Le Guyader H, Philippe H (2000) The origin of red algae and the evolution of chloroplasts. Nature 405:69–72
Matsuzaki M, Misumi O, Shin-i T et al (2004) Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 428:653–657
Collén J, Porcel B, Carré W et al (2013) Genome structure and metabolic features in the red seaweed Chondrus crispus; shed light on evolution of the Archaeplastida. Proc Natl Acad Sci 110:5247–5252
Nakamura Y, Sasaki N, Kobayashi M et al (2013) The first symbiont-free genome sequence of marine red alga, Susabi-nori (Pyropia yezoensis). PLoS ONE 8:e57122
Watanabe K, Kishimoto T, Kumagai Y, Shimizu T, Uji T, Yasui H, Kishimura H (2019) Complete sequence of mitochondrial DNA of Gloiopeltis furcata (Postels and Ruprecht). J Agardh Mitochondrial DNA B 4:2543–2544
Tu M, Liu H, Zhang R, Chen H, Mao F, Cheng S, Lu W, Du M (2018) Analysis and evaluation of the inhibitory mechanism of a novel angiotensin-I-converting enzyme inhibitory peptide derived from casein hydrolysate. J Agric Food Chem 66:4139–4144
Acknowledgements
This study was partially supported by the “Science and technology research promotion program for agriculture, forestry, fisheries and food industry (27004B)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest resulting from the contents of this article.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumagai, Y., Kitade, Y., Kobayashi, M. et al. Identification of ACE inhibitory peptides from red alga Mazzaella japonica. Eur Food Res Technol 246, 2225–2231 (2020). https://doi.org/10.1007/s00217-020-03567-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03567-z