Abstract
Braeburn variety was subjected to the process of osmotic dehydration in sucrose solution with addition of chokeberry juice concentrate. After drying by two different methods (convective- and freeze-drying), apples were stored in three different temperatures (25, 35 and 45 °C) for a period of 12 months. The aim of the study was to determine selected physical and chemical changes after storage time in each group and to show benefits of the pre-treatment use. Mass changes, water content, water activity and total colour difference ΔE, as well as anthocyanins and polyphenols content in stored dried apples were marked. It was considered that different storage conditions were statistically significant and influenced on level of changes. Storage in medium temperature (35 °C) proved to be the best efficient in terms of the lowermost values of water activity, small mass changes, as well as the smallest colour changes. Addition of chokeberry juice concentrate resulted in a significant increase of polyphenol content in dried apples. Although they were stored for a long time at different conditions, polyphenol compounds were still substantial amounts in the apple samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osmotic dehydration of fruits and vegetables is achieved by placing the plant tissue in a hypertonic solution with a simultaneous countercurrent diffusion of solutes from the osmotic solution into the tissues as well as water from the fruit to surrounding environment solution. The process affects the lower water content in the product and enables the enrichment of bio-compounds. The product obtained by osmotic process is more stable during storage due to low water activity imparted by water loss and solute gain (longer shelf life). Another advantage is the fact that sophisticated equipment is not required to carry out the dehydration [1]. Osmotic dehydration is one of the least energy-intensive processes that lead to the extend of shelf life of food, which is also attractive from the sustainable economic developments’ point of view [2]. Osmotic dehydration reduces water activity and water content of the product, which involves the inhibition of microbial growth. However, it does not affect the total preservation of the products, which require a final consolidation through the use of various drying methods [3, 4].
Dehydrated products might be used as food additives or for direct consumption. These products have many advantages, such as the ease of transportation, storage, preparation. Dried fruit produced by the use of the osmotic pre-treatment immediately before consumption or for further processing may be rehydrated [5].
In recent years, consumers’ awareness about “healthy snacks” has significantly increased and, therefore, food industry looks for interesting solutions for the food. Commercially, there is a need to create new products as well as innovations—to find answer to the question: how to reduce the cost of production. The answer may be to use the fruit as a snack in the form of dry fruit, which was previously osmo-dehydrated. The products are an alternative to high-calorie products, often lacking of nutritional value, such as sweets, traditional crisps and others. Osmotic dehydration enables to obtain new, attractive products e.g. bio-snacks. It might be seasonal fruits such as strawberries, apples in the form of fruit chips [6].
Apples are one of the most important raw materials in the Polish processing. For 12 years, Poland has been the first in Europe and the third in the world in the production of apples. The harvest in 2015 was approx. 3.5 million t (tonnes) and 1.9 million t subjected to the processing, 700 thousand t for the consumption in the country and 800 thousand t for export [7]. The main product made of apples in Poland is concentrated apple juice. The share of apples used in the production of apple juice in the total supply of the apples for processing is estimated in recent years about 90%. Participation does not show an upward trend, due to the increasing production of NFC juice (not from concentrate). However, the dried apple production has increased from approx. 0.3 thousand t in 2012 to 0.5 thousand t in 2015. The increase in production of dried apple reflects growing demand in the country and the increasing number of companies using manufacturing technologies enabling to receive product with high quality [8].
Dried fruits are rich in nutrients and they are source of energy. Consumers appreciate the presence of fiber, vitamins, as well as health properties of the product [9]. In the case of apples, the flavour is combined with a high nutritional value and health benefits. The dietary fibre, which is present in these fruits, may reduce the occurrence of many lifestyle diseases such as diabetes, atherosclerosis, cancer, cardiovascular [6]. Apples in their composition contain approx. 2–3% fibre, including the half of soluble dietary fibre (pectin) and the content of acids and sugars affect the attractiveness of the fruit. Very important compounds responsible for the biological value of the apples are polyphenolic compounds [10]. Apples are one of the main sources of flavonoids in the diet, including at least 2 g of total polyphenols per kg fresh weight or approx. 400 mg in one apple. The main groups of polyphenols in apples are flavonoids, including quercetin, (−) epicatechin, (+) catechin, procyanidin and anthocyanins; dihydrochalcones such as phloretin and phloridzin derivatives and other phenolic compounds, such as chlorogenic acid [7]. Even after convective drying apples are the source of bioactive compounds and show antioxidant activity [11]. Unfortunately, some of these compounds may be degraded during food processing and storage. Therefore, it is necessary to find preservation methods that would allow fruit to retain the nutrients that are likely to be beneficial for health to the greatest possible extent [12].
The aim of this research was to evaluate chemical and physical changes in apple tissue, using osmotic dehydration in chokeberry solution as pre-treatment before drying. The changes were marked in control and OD-samples before and after storage.
Materials and methods
Sample preparation
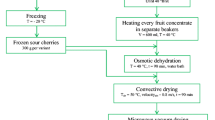
Fresh apples of the Braeburn variety were purchased from university cultivation (WULS). The fruits were stored at 4–5 °C and relative humidity of 85–90% in a refrigerator until use (2 weeks). Before each experiment, the apples were washed, peeled manually and cut into cylinders (10 mm in diameter and 10 mm in height). The samples were dipped in a solution of 1% citric acid for 10 min to prevent enzymatic browning.
Pre-treatment procedure
Fresh apples were dehydrated by osmotic dehydration (OD) at 40 °C in a water bath (Water Bath Shaker Type 357 ELPAN, Poland) with continuous shaking (1 Hz amplitude). The temperature of water bath was constant. The samples were placed into glass jars. The ratio of raw material weight to osmotic solution was maintained at 1:4 as it had previously been reported that the dilution of osmotic solution was negligible at this raw material to osmotic solution ratio [13]. Apple samples were dipped into 60oBrix solution mixtures of sucrose and chokeberry juice concentrate (CJC) (1:1) for 120 min. Afterwards samples were removed from the osmotic solution and blotted with absorbent paper to remove osmotic liquid from their surface. Two technological repetitions were performed for each treatment.
Drying
After osmotic pre-treatment, apple samples were dried by a convective or a freeze-drying equipment (Christ Gamma 1–16 LSC, Germany). Convective drying of pre-treated samples was carried out at a temperature of 70 ± 1 °C and air velocity of 1.5 ± 0.1 m/s. The dryer was loaded with 0.1 kg (1.11 kg·m− 2) of material which was spread on perforated shelf in a single layer. The air flow ran parallel to the screens and the drying process continued until constant mass was reached (approximately 7 h). Freeze-drying was performed with total pressure and temperature in a vacuum chamber equal to 100 Pa and 25 °C, respectively. Average freeze-drying time was approximately 24 h. Two technological repetitions were performed for each drying. Control samples were dried by two methods without osmotic pretreatment.
Storage
The dried apple samples were packed in plastic polyethylene bags (BOPA/PE 15/40 FF) using vacuum packing machine (PP-5.4 TEPRO, Poland) with 25% content of air inside. The weight of the dried apple inside the pack of single stored package was approximately 2.0 g. Four pieces of dried apples were packed into one package. Then they were stored in darkness at 25, 35 and 45 °C at 50–60% air humidity for 12 months. The mass changes, water content, water activity and colour parameters analysis of each of the dried products were studied.
Analytical methods
Mass changes
Dried apples were weighted at the beginning of the storage process as well as after 12 months. Mass changes of stored dried apples were showed as percentage values. Two repetitions were performed.
Water content
Water content was measured for two samples by means of drying method. The weighted material placed in a weighing glass was dried in a convective dryer (SUP-65 WG WAMED, Poland) at the temperature of 70 °C for 24 h, then in a vacuum dryer (HORYZONT SPT 200, Poland) at 60 °C for 2 h. The convective- and freeze-dried material was weighed on an analytical scale after drying at the beginning of storage and 12 months afterwards with the accuracy of 0.001 g.
Water activity
Water activity was measured using an AquaLab CX-2 (Decagon Devices Inc., USA) apparatus, in accordance with the manufacturer instruction. The temperature of water activity determination was constant (25 °C). Each measurement was conducted in 3 repetitions.
Colour measure
Colour analysis of the dried apples surface was determined with the use of Minolta Chroma Meter CR-200 (Minolta Corp., Osaka, Japan). The measurement conditions were: D65 standard illuminate, 2° Standard Observer, measurement diameter: 30 mm. The results were presented using the directly measured parameters: L* (lightness/darkness), a* (red/green), b* (yellow/blue). The measurements were made in 5 repetitions for every dried sample; the mean values are reported. Total colour difference (ΔE) was calculated according to the following formulas:
ΔL*, Δa*, Δb*—the change of L*, a* and b* parameter between dried apple samples before and after storage.
Chemical methods
Total polyphenol content
Determining the total polyphenol content was performed using the Folin–Ciocalteu’a method, as modified by Singleton and Rossi [14]. To obtain the extract for analysis, 5 g of finely ground dried fruit material was weighed with accuracy of 0.0001 g. 50 cm3 of 70% methanol was added to the material and the mixture was shaken for 1 h. The solution was then filtered through a paper filter and the supernatant extract was collected into a flask. Samples were prepared by adding 1.5 cm3 of the supernatant extract and 2.5 cm3 Folin–Ciocalteu reagent (POCH, Gliwice, Poland) to 30 cm3 of distilled water and then mixed for 3 min. with 5 cm3 of sodium carbonate solution. Next, distilled water was added to obtain 50 cm3 of total volumetric flask. Control samples, containing no extract, were prepared analogously. The solutions were kept in darkness for 1 h. Afterwards, the absorbance was measured against the blank sample (without extract) at 750 nm using a Heλios γ ThermoSpectronic (manufacturer: Thermo Spectronic, England). The determination was repeated twice for each extract. The results of total polyphenol content were expressed relative to milligrams of gallic acid per 100 g dry matter (mg GAE/100 g d.m.).
Total anthocyanin content
The total monomeric anthocyanins concentration was analyzed by the pH differential method. This is a rapid and simple spectrophotometric method based on the anthocyanin structural transformation which occurs with a change in pH (coloured at pH 1.0 and colourless at pH 4.5) [15].
To prepare extract of anthocyanins 2 g of apple was weighted into a falcon and 15 cm3 of reagent was added. The extraction reagent consisted 0.1 N hydrochloric acid and 80% ethanolic solution (15:85 v/v). The content was homogenized and shaken for 10 min with a speed equal to 2000 rpm. Magnesium carbonate was added to improve a precipitation during centrifugation which was carried out for 10 min with a velocity of 6000 rpm. After centrifugation, the supernatant was collected into a volumetric flask. This procedure was repeated thrice for the same portion of material. The volumetric flask was filled with reagent and extract used for anthocyanins determination. For anthocyanin content analysis 1.5 cm3 of extract was transferred into two glass tubes. 3.5 cm3 of buffer at pH 1.0 (0.025 M potassium chloride) was added into the first tube whereas the second tube consisted of buffer at 4.5 (0.4 M sodium acetate). The content was stirred and left in the darkness at room temperature. After 30 min of incubation, absorption was measured at 510 and 700 nm using a Heλios γ ThermoSpectronic (manufacturer: Thermo Spectronic, England). The results of total anthocyanin content were expressed relative to milligrams of cyanidin per 100 g product (mg cyanidin/100 g).
Antioxidant activity determination
The antioxidant activity was determined spectrophotometrically, based on the decrease of the solution absorbance as a result of scavenging of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma–Aldrich, USA) synthetic radical by the antioxidants present in apple tissue, according to the method specified by Wu et al. [16]. Apple extracts in 80% ethanol in the range of 0.29–2.44 mg d.m./cm3 were prepared separately in each tube. Subsequently, the 2 cm3 of 100 µM DPPH solution was added to all samples. The content was stirred and the tubes were kept in the darkness for 30 min before the spectrophotometric measurement. The absorbance was read at 515 nm against 80% ethanol. The antioxidant potential of the apples tissue was determined in four independent replications and was expressed as DPPH50 parameter, which means a concentration of apple tissue in extract which allows to scavenge a 50% of DPPH radical (in mg d.m./cm3).
Statistical analysis
The statistical software Statgraphics Plus ver. 5.1 (StatPoint) and Excel 2016 (Microsoft) were used for data analysis. The influence of osmotic pre-treatment on achieved values was evaluated by t test to compare the means of the two samples (control and after osmotic dehydration). The test has been constructed to determine whether the difference between the two means equals 0.0 versus the alternative hypothesis that the difference does not equal 0.0. In the case when the computed P value had been less than 0.05, we rejected the null hypothesis. Pearson’s correlation coefficient between water activity and water content was calculated. The influence of storage conditions (drying method, storage temperature) on dependent variables: mass changes, the water content, water activity and colour values, was evaluated by means of a two factorial analysis of variance (ANOVA) at a significance level α = 0.05. In the case of significant impact factor post-hoc Tukey’s test was performed. Homogenous groups were marked on the Figures (a—method of drying, A—storage temperature).
Results and discussion
Mass changes
The storage result of dried apple samples at constant temperatures of 25, 35 and 45 °C during 12 months was satisfactory. The changes in the mass of apples were small. It was noted that the samples stored at room temperature (25 °C) were characterized by a slight increase in mass (2–4%) what could be caused from the absorption of moisture from the air. Meanwhile, in the cases of the samples stored in higher temperatures decrease in mass between 1 to 5% was observed (Fig. 1). The highest storage temperature (45 °C) resulted in the highest values of weight loss compared to those stored at lower temperatures. This probably resulted from the evaporation of some water from the product. The convective dried samples had higher weight losses compared to those obtained by freeze-drying (Fig. 1). Statistical analysis of two-samples comparison (dried apples stored at these same conditions, before and after pre-treatment) showed no significant difference between the means of the two samples at the 95.0% confidence level (P value = 0.448—no reason to reject the null hypothesis that compared means are equal). Despite low mass changes, two-factor analysis of variance showed a significant difference between the achieved values of the mass changes. The differences regarded all of the factors: drying method and storage temperature (Table 1).
Water content
Water content in fresh apple variety Braeburn in this research was approximately 86.5% and it was similar to Piasecka et al. [12] research. As a result of freeze-drying, the water content of the dried material was reduced to the value of 1.6%, while in the case of convective-drying the achieved value was of about 3.2%. The use of osmotic pre-treatment allowed to receive even less value of water content after convection (approx. 2.4%) and comparable value after freeze-drying (approx. 2%). However, statistical analysis did not confirm significant impact of osmotic dehydration on achieved values of the water content (P value = 0.589). Cichowska and Kowalska [17] in previous research reported that kind of osmotic agent used as pre-treatment before drying and storage had significant influence on this parameter in contrast to temperature of the process. Klewicki et al. [18] also observed a lower water content in the freeze-dried apples (approx. 0.4%), compared to apples received by convection (approx. 7.6%).
Surprisingly, drying method had no significant effect on the water content in dried fruits, but the effect of storage temperature was observed (Table 2). Mostly in the scientific papers, significant influence of drying method was reported. For example Cichowska and Kowalska [17] also observed that storage time had no significant effect on moisture content. Room conditions proved to be the least efficient. In this case, the samples showed the highest water content (approx. 5–6%), regardless of the pretreatment in the solution of concentrated chokeberry juice (Fig. 2). Dried samples which were stored at 25 °C in both group—control and after pre-treatment—contained three times more water comparing to these samples which were stored at another temperatures. Storage during 12 months resulted in changes of water content in the samples. Freeze-dried samples stored at the lowest temperature had considerable increase of values (till 6%), whilst convective dried samples had less significant increase of values (comparing to the output value before storage). It was noticed that freeze-dried samples, which were stored at medium temperature did not show changes in water content. However, convective dried samples and stored in the same condition behaved differently—after storage they had decrease in water content. The lowest water content was noticed in osmotic treated samples, which were stored at 45 °C—achieved a value below 1%. In Rizzolo et al. [19] studies water content prior to storage in the apples was at 1.6% and also decreased as a result of storage.
Water activity
At room temperature, osmo-dehydrated food remains stable up to 6 months and even 1 year. At low water activity level, the chemical reactions as well as growth of toxin producing microorganisms are ceased [20]. The water activity is an important parameter that affects the microbial safety and is highly important for the shelf life of osmo-dehydrated products. It is defined as the available moisture content of a food product [3]. Different microbes need various levels of water activity for their growth. Proliferation of microorganism ceased when the water activity is ≤ 0.5. However, it was presumed that microbiological safety was ensured when the water activity values were ≤ 0.6 [21].
Dried samples stored during 12 months in all analyzed conditions preserved microbiological stability. The water activity values of the samples did not exceed the value of 0.350 (Fig. 3). At the start of the storage control samples exhibited water activity of 0.132 and 0.246 (in the case of using freeze- and convective drying, respectively). After osmotic dehydration and drying process, achieved values were higher: 0.180 and 0.270, respectively. Similar to previous parameter (water content), in dried apple samples stored at 25 °C increase values of water activity during storage was observed. Moreover, those values were about twice higher compared to achieved during storage in another conditions (Fig. 3). Storage at higher temperatures resulted in decrease of water activity values in almost all cases. Only freeze-dried apples from control group had minor increase of value at 45 °C and at medium temperature did not show changes in water activity. Medium storage temperature (35 °C) conditions proved to be the best efficient. In this case, the samples showed the lowermost water activity. Slightly lower values of the parameter were achieved with the use of freeze drying method, compared with the convection drying. Klewicki et al. [18] also confirmed in their research that freeze-dried apples and pre-dehydrated ones had lower values of water activity in comparison to convective samples.
Statistical analysis confirmed the significance of the two variable factors (Table 3). However, the process of osmotic dehydration was not significant on achieved values of water activity (P value = 0.870). Since the computed P value was not less than 0.05, we could not reject the null hypothesis. In addition, Cichowska and Kowalska [17] reported insignificant influence temperature and kind of osmotic agent on achieved values. Whereas Ciurzyńska et al. [22] observed significant effect of time, temperature and a type of the osmotic substance on water activity.
Pearson’s correlation coefficient between water activity and water content was calculated separately for convective and freeze-dried samples. In both cases, strong linear relationship between the variables was stated. The correlation coefficient was 0.96 for dried apples, which were obtained by convective and 0.86 for freeze-dried samples. The significant relationship between the variables proves the fact that changes of water activity depend primarily on changes of water content. It is worth mentioning that apples contain sugars (fructose, sucrose etc.), so during long storage some of them could crystallized thus increased the water activity [23]. This same relationships observed Cichowska and Kowalska [17].
Change in colours
Under the influence of the technological processes applied the change in the appearance of the samples of dried apples occurred. The samples immersed in sucrose containing chokeberry juice concentrate were darker, due to the dark colour of the solution. As a result of convective tissue shrinkage, a decrease in their lightness were observed. Freeze-drying allowed to maintain the appearance similar to that of the raw material in terms of colour and shape. However, under the influence of a long period of storage, the change of colour was observed. Control and osmotic dehydrated samples were compared with the colour of dried fruit obtained by sublimation and convection, respectively, before storage. In control group, higher storage temperature resulted more changes of colour. Freeze-dried samples were characterized of higher ΔE comparing to convective dried samples (Fig. 4). Opposite situation was observed in the case of osmo-dehydrated group. Freeze-dried samples better kept colour during storage. As a result of convective drying tissue contraction at higher temperature was followed by a fruit, and darkening of colour. It is assumed that enzymatic and non-enzymatic reactions are responsible for the degradation [24]. Statistical analysis did not confirm influence of drying method and classified values into one homogenous group (Table 4). There was also no significant difference between storage at temperatures 25 and 35 °C.
The most value of total colour difference in the case of untreated by osmosis (control samples) freeze-dried fruit stored at 45 °C was observed. Probably, it was related to the growth of water content after storage. Colour change of dried samples was due to Maillard reaction, enzymatic browning and ascorbic acid oxidation [25].
Total polyphenol content
Osmotic dehydration, drying method and storage temperature had significant effect on the changes in polyphenol content (P < 0.005). In fresh and osmodehydrated apple it equalled to 242 ± 5 and 590 ± 14 mg GAE /100 g d.m., respectively. After freeze and convective drying, polyphenol content in apple decreased by approximately 6 and 60%, respectively (Table 5). This behaviour agree with those reported in other products. Total polyphenol content significantly decreased after the convective drying, e.g. in garlic [26] and jujubes [27]. Wojdyło et al. [28] reported a 5% loss of total polyphenol content in freeze-dried strawberries of Kent variety, but for Elsanta polyphenol its value was comparable to that of the fresh fruits. Addition of the chokeberry juice to the sucrose solution during osmotic pre-treatment resulted in a significantly higher content of polyphenol compounds, amounted to 245 ± 15 mg GAE/g d.m. in osmo-convective dried sample and 598 ± 14 mg GAE/g d.m. in osmo-freeze dried sample (Table 5). The degradation of polyphenol compounds during convective drying can also be affected by cellular destruction, which is due to high temperature and long drying time [29]. The freeze-drying has some advantages, such as morphological preservation and biochemical properties in compared to other drying methods [30]. These advantages have resulted from oxygen-poor atmosphere and low temperature during this drying [30, 31].The highest quality of dried products can be obtained using freeze-drying, but this process is relatively expensive and slow [32,33,34,35]. After 12 months of storage at 25, 35 and 45 °C, polyphenol content in osmo-convective dried apple decreased by approximately 48, 59 and 72%, respectively.
In the case of samples which were osmotically dehydrated before freeze drying, a significant decrease in the total amount of polyphenol compounds was observed. After storage at 25, 35 or 45 °C, osmo-freeze died sample contained approximately 40, 47 and 75% less polyphenols than before storage, respectively.
Dehydrating apples in chokeberry juice concentrate enriched them in polyphenols. Despite the fact the samples were stored for a long time (12 months) in different conditions, substantial amounts of polyphenol compounds were still present, especially in osmo-freeze dried samples. However, low selectivity of the Folin–Ciocalteu’s method should be taken into account here; factors, for example, the presence of ascorbic acid could influence the result [36].
Polyphenol compounds play an important protective function in the fruit [37]. Piasecka et al. [12] determined the stability of polyphenol compounds and ascorbic acid in osmotically dehydrated fruit, which were subsequently convectively or freeze-dried, and stored for 12 months. In the research apples, cherries and blackcurrants were dehydrated in the following osmotic solutions: apple juice and sour cherry juice, fructooligosaccharide concentrate, trehalose solution, sucrose and inverted sugar.
The authors [12] observed that the content of polyphenol compounds in fresh apples was approx. 188 mg/100 g. Other results were obtained by Sacchetti et al. [38]. They observed that polyphenol content was in the range of 221–530 mg/100 g depending on the apple variety. Different drying processes had impact on the concentration of polyphenol compounds in apple tissue [39]. In the case of convectively dried material, a gradual decrease in total phenolic content was observed during storage. In freeze-dried apples which were beforehand osmo-dehydrated in sucrose, polyphenol contents were also relatively stable. They changed only by 27% in 12 months. In the case of freeze-dried apples, the HPLC analysis showed that the main polyphenol compound was chlorogenic acid [12]. They showed that higher fluctuations in polyphenol contents occurred in apples osmo-dehydrated in sour cherry concentrate prior to freeze-drying. The initial levels were higher (783 mg epicatechin equivalent (ECE)/100 g) than those of fruits soaked in sucrose (441 mg ECE/100 g) as a result of the diffusion of polyphenol from the osmotic solution into fruit tissue. After 12 months storage, 61% of initial polyphenol remained in the product. In the research presented by Piasecka et al. [12] polyphenols in freeze-dried fruits were much more stable than in convectively dried materials. Blackcurrants retained almost 80% of polyphenols after 12 months of storage, sour cherries about 70% and apples 60–70%.
Anthocyanins content
The analysis has shown that storage affects the concentration of anthocyanins in apple tissue. Generally, results indicated that storage contributed to a decrease of the total content of anthocyanins in apple tissue and the size of the decrease did not depend on storage temperature (Table 5). In the study, anthocyanin content in fresh, convective- and freeze-dried apple without OD was 0 mg of cyanidin/100 g. Osmotic dehydration combined with lyophilization or air drying caused an increase in the concentration of the above-mentioned substances. After 12 months storage at 45 °C, a substantial decrease in the concentration of anthocyanins was observed for osmo-convective dried apple (from 98 ± 6 to 4 ± 1 mg of cyanidin/100 g) and osmo-freeze dried apple (from 309 ± 34 to 26 ± 2 mg of cyanidin/100 g). The loss in anthocyanins amounted to above 90%, regardless of the drying method. The destruction of dyes increases with increasing temperature of storage. There occurs the conversion from red anthocyanin cation to colorless or yellow chalcones, and then brown polymers [40]. Czapski and Walkowiak-Tomczak [41] were noted analogous results were obtained in the research. Researchers demonstrated the effect of heating on the stability of chokeberry anthocyanins. In addition, Ścibisz et al. [40] observed a similar dependency in blueberries. It was found that with increasing temperature and time, the parameters L* and b* values increased as well.
DPPH 50 assay
In dried apples after storage time the DPPH 50 were significant changed. Antioxidant activity measured by DPPH 50 radical scavenging decreased throughout the storage time for all the studied samples (Table 2). The DPPH 50 equalled to 3.12 ± 0.08 mg d.m./cm3 in convective dried apple, 1.41 ± 0.14 mg d.m/cm3 in freeze-dried apple, 2.14 ± 0.11 mg d.m./cm3 in osmo-convective dried sample and 0.67 ± 0.09 mg d.m./cm3 in osmo-freeze dried sample. Eim et al. [42] and Rodríguez et al. [43] observed that convective drying may cause a decrease in antioxidant activity. The storage temperature had no significant effect on antioxidant activity after 12 months of storage. In general, DPPH is correlated with the amount of polyphenols present in plant tissue. Ismail et al. [44] studied the DPPH scavenging activity of cantaloupe extracts and found a good relation with total polyphenol content (r2 = 0.9228).
Conclusions
This study confirmed that dried apples after 12-months storage in different conditions still remain microbiologically stable. However, long storage at 25 °C was not efficiently due to increase of water content as well as water activity. Drying method had no significant effect on the water content and total colour difference, whereas the effect of storage temperature was observed. The best conditions for the storage of dried apples were at medium temperature (35 °C). In this case, the smallest mass and colour changes were noticed, and water activity after 12-months storage was the lowermost. Osmotic dehydration as pre-treatment before drying did not reduce changes during storage, but had influence on chemical changes in apple tissue. Dehydrated samples in chokeberry juice concentrate had been enriched with polyphenols. Although they were stored for a long time at different conditions, polyphenol compounds were still substantial amounts in the apple samples. Freeze-drying was better method of drying in terms of higher values of phenolic as well as anthocyanins content before and after storage.
References
Sutar N, Sutar PP (2013) Developments in osmotic dehydration of fruits and vegetable-a review. Trends Post Harvest Technol 1(1):20–36
Astyk S (2009) In: Astyk S (ed) Dehydration, independence days: a guide to sustainable food storage & preservation. New Society Publishers, Canada, 177–190
Ahmed I, Quazi IM, Jamal S (2016) Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov Food Sci Emerg 34:29–43
Yadav AK, Singh SV (2012) Osmotic dehydration of fruits and vegetables: a review. J Food Sci Technol Doi. https://doi.org/10.1007/s13197-012-0659-2
Rastogi NK, Nayak CA, Raghavarao KSMS. (2004) Influence of osmotic pre-treatments on rehydration characteristic of carrots. J Food Eng 65:287–288
Chang SK, Alasalvar C, Shahidi F (2016) Review of dried fruits: Phytochemicals, antioxidant efficacies, and health benefits. J Funct Food 21:113–132
Achremowicz B, Oszmiański J (2016) Fruit and vegetables antioxidants. Part II. Apples antioxidants. Ferment Fruit Veg Process Ind 04:22–26. https://doi.org/10.15199/64.2016.4.3
Nosecka B, Trojanowicz P (2016) The demand for apple of processing plants in Poland. Ferment Fruit Veg Process Ind 04:10–13. https://doi.org/10.15199/64.2016.4.1
Jesionkowska K, Sijtsema SJ, Konopacka D, Symoneaux R (2009) Dried fruit and its functional properties from a consumer’s point of view. J Hortic Sci Biotech 84(6):85–88
Raskin I, Ripoll C (2004) Can an apple a day keep the doctor away? Curr Pharm Des 3:1381–1392
Wojdyło A, Figiel A, Oszmiański J (2007) Influence of temperature and time of apple drying on phenolic compounds content and their antioxidant activity. Pol J Food Nutr Sci 57(4(C)):601–605
Piasecka E, Uczciwek M, Klewicki R, Konopacka D, Mieszczakowska-Frąc M, Szulc M, Bonazzi C (2013) Effect of long-time storage on the content of polyphenols and ascorbic acid in osmo-convectively dried and osmo-freeze-dried fruits. J Food Process Pres 37:198–209
Oliveira FIP, Gallao MI, Rodriguez S, Fernandes FAN (2011) Dehydration of Malay apple (Syzygiummalaccense L.), using ultrasound as pre-treatment. Food Bioprocess Technol 4:610–615
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Giusti MM, Wrolstad RE (2001) In: Wrolstad RE (ed) Anthocyanins. Characterization and measurement with UV-visible spectroscopy. Current Protocols in food analytical chemistry. Wiley, New York F1.2.1–1.2.13
Wu CR, Huang MY, Lin YT, Ju HY, Ching H (2007) Antioxidant properties of Cortex Fraxini and its simple coumarins. Food Chem 104:1464–1471
Cichowska J, Kowalska H (2018) Effect of osmotic pre-treatment and temperature storage conditions on water activity and colour of dried apple. Int J Food Eng 14(2):20170158 (ISSN (online) 1556–3758)
Klewicki R, Konopacka D, Uczciwek M, Irzyniec Z, Piasecka E, Bonazzi C (2009) Sorption isotherms for osmo-convectively-dried and osmo-freezedriedapple, sour cherry, and blackcurrant. J Hortic Sci Biotech 84(6):75–79 (ISAFRUIT Special Issue)
Rizzolo A, Vanoli M, Cortellina G, Spinelli L, Torricelli A (2011) Quality characteristics of air-dried apple rings: influence of storage time and fruit maturity measured by time-resolved reflectance spectroscopy. Proc Food Sci 1:216–223
Ahmed J, Choudhary DR (1995) Osmotic dehydration of papaya. Indian Food Packer 49:5–11
Prothon F, Ahrne LM (2004) Application of the Guggenheim, Anderson and De Boermodel to correlate water activity and moisture content during osmotic dehydration of apples. J Food Eng 61:467–470
Ciurzyńska A, Cichowska J, Kowalska H, Czajkowska K, Lenart A (2018) Osmotic dehydration of Braeburn variety apples in the production of sustainable food products. Int Agrophys 32:141–146
Yu X, Kappes SM, Bello-Perez LA, Schmidt SJ (2008) Investigating the moisture sorption behavior of amorphous sucrose using a dynamic humidity generating instrument. J Food Sci 73:E25–E35
Schulze B, Hubbermann EM, Schwarz K (2014) Stability of quercetin derivatives in vacuum impregnated apple slices after drying (microwave vacuum drying, air drying, freeze drying) and storage. LWT-Food Sci Technol 57:426–433
Udomkun P, Nagle M, Argyropoulos D, Mahayothee B, Latif S, Müller J (2016) Compositional and functional dynamics of dried papaya as affected by storage time and packaging material. Food Chem 196:712–719
Calín-Sanchez A, Figiel A, Wojdyło A, Szarycz M, Carbonell-Barrachina ÁA (2014) Drying of garlic slices using pre-drying and vacuum-microwave finishing drying: Kinetics, energy consumption, and quality studies. Food Bioproc Tech 7:398–408
Wojdyło A, Figiel A, Legua P, Lech K, Carbonell-Barrachina ÁA, Hernández F (2016) Chemical composition, antioxidant capacity, and sensory quality of dried jujube fruits as affected by cultivar and drying method. Food Chem 207:170–179
Wojdyło A, Figiel A, Oszmiański J (2009) Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J Agric Food Chem 57:1337–1343
Alean J, Chejne F, Rojano B (2016) Degradation of polyphenols during the cocoa drying process. J Food Eng 189:99–105
Liapis AI, Bruttini R (2007) Freeze drying. In: Mujumdar AS (ed) Handbook of industrial drying, 3rd edn. Taylor and Francis Group, New York, pp 257–281
Koroishi ET, Boss EA, Maciel MRW, Filho RM (2009) Process development and optimization for freeze-drying of natural orange juice. J Food Process Eng 32:425–441
Hsu CL, Chen W, Weng YM, Tseng CY (2003) Chemical composition, physical properties and antioxidant activities of yam flours as affected by different drying methods. Food Chem 83:85–92
Khalloufi S, Robert JL, Ratti C (2005) Solids foods freeze-drying simulation and experimental data. J Food Process Eng 28:107–132
Marques LG, Silveira AM, Freire JT (2006) Freeze-drying characteristics of tropical fruits. Dry Technol 24:457–463
Rozylo R, Rudy S, Krzykowski A, Dziki D (2015) Novel application of freeze-dried amaranth sourdough in gluten-free bread production. J Food Process Eng 38:135–143
Polovka M, Brezova V, Stasko A (2003) Antioxidant properties of tea investigated by EPR spectroscopy. Biophys Chem 106:39–56
Mikulic-Petkovsek M, Schmitzer V, Slatnar A, Stampar F, Veberic R (2012) Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J Food Sci 10:1064–1070
Sacchetti G, Cocci E, Pinnavaia GG, Mastrocola D, Dalla Rosa M (2008) Influence of processing and storage on the antioxidant activity of apple derivatives. Int J Food Sci Tech 43:797–804
Joshi APK, Rupasinghe HPV, Khanizadeh S (2011) Impact of drying processes on bioactive phenolics, vitamin C and antioxidant capacity ofred-fleshed apple slices. J Food Process Pres 35:453–457
Ścibisz I, Kalisz S, Mitek M (2010) Thermal degradation of antocyanins in blueberry fruit. Żywność: Nauka Technologia Jakość 5:56–66
Czapski j, Walkowiak-Tomczak D (2008) Kinetics of anthocyanin colour changes during heating solutions of chokeberry, enocyanin, enocyanin and elderberry pigments. Acta Agroph 12:625–636
Eim VS, Urrea D, Rosselló C, García-Pérez JV, Femenia A, Simal S (2013) Optimization of the drying process of carrot (Daucus carota v. Nantes) on the basis of quality criteria. Dry Technol 31(8):951–962
Rodríguez Ó, Santacatalina JV, Simal S, Garcia-Perez JV, Femenia A, Rosselló C (2014) Influence of power ultrasound application on drying kinetics of apple and its antioxidant and microstructural properties. J Food Eng 129:21–29
Ismail HI, Chan KW, Mariod AA, Ismail M (2010) Phenolic content and antioxidant activity of cantaloupe (cucumismelo) methanolic extracts. Food Chem 119:643–647
Acknowledgements
This work was financially supported by SUSFOOD ERA-Net / NCBiR (National Centre for Research and Development); Project No 5/SH/SUSFOOD1/2014. Implementation period: 2014–2016, Poland. The work was also co-financed by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Food Sciences of Warsaw University of Life Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with Ethics requirements
This article does not contain any studies with human or animal subjects.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cichowska, J., Samborska, K. & Kowalska, H. Influence of chokeberry juice concentrate used as osmotic solution on the quality of differently dried apples during storage. Eur Food Res Technol 244, 1773–1782 (2018). https://doi.org/10.1007/s00217-018-3089-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3089-1





 25 °C Freeze-drying,
25 °C Freeze-drying,  25 °C Convection drying,
25 °C Convection drying,  35 °C Freeze-drying,
35 °C Freeze-drying,  35 °C Convection drying,
35 °C Convection drying,  45 °C Freeze-drying,
45 °C Freeze-drying,  45 °C Convection drying
45 °C Convection drying

