Abstract
The impact of single- and two-step enzymatic modification of wheat flour proteins was investigated on their immunoreactivity. Modification of wheat proteins was conducted using preparations of prolyl endopeptidase, transglutaminase and peptidases synthesised by Lactobacillus acidophilus 5e2 and L. sanfranciscensis DSM20663. Hydrolysis of cross-linked proteins and cross-linking of proteins’ hydrolysates was used during two-step modification. Immunoreactivity of wheat flour proteins was decreased with the increase in environmental alkalinity after treatment with transglutaminase. These modifications resulted in changes in protein composition and average polypeptide chain length. Fractional composition of gliadins demonstrated that decreasing acidity of reaction medium is associated with more effective cross-linking of ω-gliadins. Protein hydrolysis by prolyl endopeptidase and peptidases from lactobacilli also resulted in effectively degrading of HMW-glutenins and ω-gliadins. The lowest content of the immunoreactive gliadins was observed for proteins degraded with peptidases. Immunological analysis of proteins subjected to two-step enzymatic modification also showed a reduction in the content of gliadins both in the samples of cross-linked protein hydrolysates, and in cross-linked proteins. Hydrolysis of cross-linked proteins favoured more the reduction in protein immunoreactivity than cross-linking of hydrolysed proteins. Wheat proteins undergo the most effective modifications favouring the reduction in immunoreactivity, when transglutaminase/peptidase LS and peptidase LS/transglutaminase combinations were used. Proposed methods of enzymatic reduction in gluten immunoreactivity could be used as additional step of flour modification in sourdough technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coeliac disease is a chronic disorder of the small intestine leading to villous atrophy and crypt hyperplasia, occurring both in children and adults. It is estimated that it affects 1–3 % of the human population. Its development is triggered by the consumption of gluten proteins derived from wheat, rye or barley by genetically predisposed individuals [1, 2].
Peptides released from gluten rich in proline and glutamine play a key role in the aetiology of coeliac disease. Proline due to its cyclic side chain affects the secondary and tertiary structure of peptides and proteins, conditioning their biological properties. In the polypeptide chain, it is responsible for the formation of β-turns and creates a structure which is characterised by a higher spatial density than α-helix [3]. The presence of adjacent proline residues in the amino acid sequence makes peptide bonds resistant to proteolytic enzymes of the human gastrointestinal tract and helps them to penetrate the intestinal barrier [4, 5]. Moreover, the presence of glutamine residues makes them a good substrate for a reaction with tissue transglutaminase (tTG) [6]. Deamidation of glutamine to glutamic acid increases the affinity of peptides to antigens of the major histocompatibility complex MHC II (HLA-DQ2) [7]. In addition, some of the peptides isolated from γ-gliadins and glutenins exhibit low immunogenicity in their native form, but they become immunodominant and strongly influence T cells as a result of deamination involving TG [8, 9].
In coeliac aetiology, there are essential gluten peptides which are toxic and/or immunogenic. The peptide if defined as immunogenic if it is able to specifically stimulate T cells derived from jejunal mucosa or peripheral blood of coeliac patients. In turn, toxic peptides are able to induce mucosal damage [10]. In patients with coeliac disease, T cells are stimulated with peptide fragments of gliadins, e.g., α(93–106), γ(60–79), γ(102–113), whose sequences demonstrate a high content of proline and glutamine [8, 9]. Peptides with sequences LGQQQPFPPQQPYPQPQPF, PQPELPYPQPQLPY, QLQPFPQPQLPYPQPQS isolated from α-gliadin are toxic [11]. Peptides damaging the epithelial cells in people with coeliac disease under in vitro conditions comprise in their molecular structure one of the amino acid sequence motifs including: PSQQ, QQQP, QQPY or QPYP, which also contain proline and glutamine [12]. The enzyme-linked immunosorbent assay (ELISA) can identifying immunogenic or toxic peptides or their modified forms using monoclonal R5 antibody. The R5 antibody recognised sequences QQPFP, LQLQPFP, QLPYP, PQPF and PQPFP which are present in immunogenic and/or toxic gluten peptides [13]. Elimination of the immunogenic potential of such peptides is based on the degradation or modification of the structure of the epitopes recognised by the cells of the immune system, using proline- or glutamine-specific enzymes. The enzymes applied for this purpose include: prolyl endopeptidase (PEP, EC 3.4.21.26), transglutaminase (TG, EC 2.3.2.13), peptidases synthesised by lactic acid bacteria (LAB), peptidases synthesised by fungi of the Aspergillus genus and endogenous cereal peptidases [14–20].
An alternative to the gluten-free diet is the elimination of coeliac toxic and immunogenic peptide fragments at the stage of food production or by supporting the digestion in the human gastrointestinal tract [20–23]. Studies on the degradation of gluten by oral enzyme therapy are focused on the use of bacterial prolyl endopeptidases from Flavobacterium meningosepticum, Myxococcus xanthus and Sphingomonas capsulata [24], prolyl endopeptidase from Aspergillus niger [25] and cysteine endopeptidase from germinating barley grains (EP-B2) [16].
The use of LAB as a source of proteolytic enzymes promotes the degradation of gluten proteins as early as at the stage of bread production [19]. Bacteria isolated from sourdough including: Lactobacillus alimentarius 15M, L. brevis 14G, L. sanfranciscensis 7A and L. hilgardii 51B exhibit the ability to degrade a coeliac toxic 33-mer polypeptide which is resistant to enzymatic digestion of the human gastrointestinal tract. These strains exhibit the enzymatic activity characteristic of iminopeptidases, dipeptidyl peptidases, prolyl endopeptidases, prolidases, prolinases and aminopeptidases [21].
The combination of the activity of LAB and fungal peptidases, conventionally used in bakery technology, in the sourdough, promotes the degradation of gluten to a level below 10 mg/kg [19]. Peptidases of fungal origin hydrolyse native proteins to peptides with a length of 4–40 amino acids, which are successively transported into the bacterial cells and become a substrate for intracellular peptidases [26]. The fermentation of wheat flour using LAB and peptidases from A. oryzae and A. niger reduces immunoreactivity of gliadins and glutenins by 66 and 20 %, respectively. The flour prepared in this way, which is subsequently subjected to digestion with pepsin and trypsin exhibits an eightfold lower ability to agglutinate K562(S) cells, compared to native flour [22].
In turn the use of TG, depending on the conditions of the reaction medium, may either result in the formation of new epitopes due to deamidation, or mask the epitopes which are already present in the peptides as a result of transamidation [27]. Stimulation of T cells with immunoreactive α(56–68) peptide obtained from gliadins and a pepsin and trypsin hydrolysate of gliadins (PT-gliadin), containing numerous immunoreactive domains, leads to the release of γ-interferon. The obtained α(56–68–Q65–K–CH3) peptides, as a result of transamidation with methyl esters of lysine, inhibit secretion of γ-interferon by T cells collected from patients with diagnosed coeliac disease. After transamidation the modified peptide loses its ability to bind to HLA-DQ2 molecules. In contrast, the native α(56–68) peptide, as well as the α(56–68–E65) peptide subjected to deamidation, binds to HLA-DQ2 and strongly stimulates T cells. PT-gliadins have similar immunological properties as the study peptide [28].
Gluten was also modified by using initial hydrolysis by subtilisin or collagenase and cross-linking of hydrolysates by chemical reagents. Wheat flour gliadins’ immunoreactivity was decreased to 3.3 and 4.6 % when, respectively, subtilisin and collagenase ware used following polyethylenimine cross-linking [29]. In contrast, there is still lack of scientific data on influence of two-step wheat flour protein modification using proteolytic enzymes and transglutaminase on their properties.
The aim of the study is to determine the effect of a single- and two-step enzymatic modification of wheat proteins on their immunoreactive properties.
Materials and methods
Materials
The strain of lactic acid bacteria L. acidophilus 5e2 (Rhodia Food Biolacta, Olsztyn, Poland) and L. sanfranciscensis DSM20663 (Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) were kept frozen at −35 °C on MR-broth (Merck, Darmstadt, Germany) with the addition of glycerol (25 % v/v). Before the experiment, the bacteria were double-passaged on MRS-broth for 12 h at a temperature of 30 or 37 °C for L. sanfranciscensis DSM20663 and L. acidophilus 5e2, respectively.
Enzyme preparations of prolyl endopeptidase (PEP, Brewers Clarex, DSM Food Specialties, Delft, Holand), transglutaminase (TG, Activa WM, Ajinomoto Foods Deutschland GmbH, Hamburg, Germany) and wheat flour (type 650, Młyny Szczepanki, Łasin, Poland) were also used in all experiments.
All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO USA).
Biosynthesis and isolation of intracellular peptidases
A sterile MRS-broth (1800 mL) supplemented with gluten (1 % w/v, Sigma G5004) was inoculated with a 12 h culture of L. acidophilus 5e2 and L. sanfranciscensis DSM20663 bacteria (10 % v/v), and a stationary culture was run without aeration at a temperature of 30 or 37 °C for 12 h. The LAB biomass was separated from the post-culture fluid by centrifugation (5000×g, 20 min, 5 °C, 3–18 K Sigma, Laborzentrifugen GmbH, Oserode am Harz, Germany), double-rinsed with a sterile physiological solution (20 mL, NaCl, 0.8 % w/v), each time separating the biomass by centrifugation (5000×g, 20 min, 5 °C). Next, biomass (10 g) was suspended in a sterile physiological solution (100 mL, NaCl, 0.8 % w/v) and subjected to ultrasound disintegration (pulse on: 4 s; pulse off: 1 s; 5 °C, 30 min; VCX500 Vibre Cell, Sonics, Newtown, CT, USA). The resultant homogenate was centrifuged (1000×g, 15 min, 5 °C) in order to separate the fragments of damaged cell walls. The supernatant containing peptidases was fixed by means of lyophilisation (Ralph 1-2 LD, Christ GmbH, Osterode am Harz, Germany), thus obtaining a preparation of intracellular enzymes.
Enzymatic modification of wheat grain proteins
Modification of wheat proteins was conducted at 30 °C for 3 h using the doses of enzymes of 10 U/g of flour. In the experiment, enzymatic preparations of PEP, TG, and peptidases synthesised by L. acidophilus 5e2 (LA) and L. sanfranciscensis DSM20663 (LS) were used. Due to the diversity of peptidases synthesised by lactic acid bacteria, the addition of LA and LS was determined based on the activity of endopeptidases.
Cross-linking of proteins
Wheat flour (1.5 g) was suspended in phosphate buffers (3 mL, 6.6 mmol/L, pH 5.0, pH 6.0, pH 7.0) and Tris–HCl buffer (3 mL, 0.2 mol/L, pH 8.5) containing TG (5 U/mL) and subjected to incubation (3 h, 30 °C) with stirring. Samples of the modified flour were heat treated (15 min, 95 °C) to inactivate TG, frozen and freeze-dried (Ralph 1-2 LD, Christ GmbH, Osterode am Harz, Germany).
Hydrolysis of proteins
Wheat flour (1.5 g) was suspended in water (3 mL) containing PEP, LA or LS preparations (5 U/mL) and subjected to incubation (3 h, 30 °C) with stirring. Samples of the modified flour were heat treated (15 min, 95 °C) to inactivate enzymes, frozen and freeze-dried.
Hydrolysis of cross-linked wheat proteins
Wheat flour (1.5 g) was suspended in water (1.5 mL) containing TG (10 U/mL) and subjected to incubation (1.5 h, 30 °C) with stirring. Then, aqueous solutions of PEP, LA or LS preparations (1.5 mL, 10 U/mL) were introduced into the reaction medium. All samples were subjected to incubation (1.5 h, 30 °C) with stirring. Finally, the samples of protein hydrolysates were heated (15 min, 95 °C) to inactivate enzymes, frozen and freeze-dried.
Cross-linking of wheat protein hydrolysates
Wheat flour (1.5 g) was suspended in water containing PEP, LA or LS preparations (1.5 mL, 10 U/mL) and subjected to incubation (1.5 h, 30 °C) with stirring. Then, TG suspended in water was introduced into the reaction medium (1.5 mL, 10 U/mL). The samples were subjected to incubation (1.5 h, 30 °C) with stirring. Finally, the samples of protein hydrolysates were heated (15 min, 95 °C) to inactivate enzymes, frozen and freeze-dried.
For samples of native and modified proteins, the degree of protein hydrolysis, average polypeptide chain length and protein immunoreactivity were determined, and gliadins were isolated and characterised by electrophoresis.
Determination of the activity of enzyme preparations
Determination of the endopeptidase activity
The analysed intracellular enzymatic preparations (50 mg) obtained from L. acidophilus 5e2 and L. sanfranciscensis DSM20663 were suspended in a McIlvaine buffer (1 mL, 0.2 mol/L, pH 7.0) and supplied with an azocasein substrate (1.4 mL, 1.4 % w/v in McIlvaine buffer). The reaction mixture was incubated for 3 h at a temperature of 30 °C with stirring. The reaction was stopped by the addition of trichloroacetic acid (2 mL, 10 % w/v, 7 °C). Supernatant was separated by centrifugation (8000×g, 10 min, 21 °C), and the resultant supernatant (1 mL) was mixed with an NaOH solution (1 mL, 0.5 mol/L) and incubated (20 min, 30 °C). Afterwards, sample absorbance was measured at a wavelength of 440 nm (DU-650, Beckman). The unit of endopeptidase activity was defined as increase in sample absorbance of 0.01 at 440 nm within 1 h at 30 °C and pH 7.0.
Determination of the proline peptidases activity
The activity of proline endopeptidase was determined using synthetic substrates: Z-Gly-Pro-pNa, Gly-Pro-pNa, Arg-Pro-pNa and Pro-pNa at a concentration of 20 mmol/L in methanol. The intracellular enzymatic preparation (50 mg) was dissolved in a phosphate buffer (2.95 mL, 6.6 mmol/L, pH 7.0), and the substrate was then added (50 μL). The reaction mixture was incubated for 1 h at a temperature of 30 °C with stirring. The reaction was stopped by the addition of acetic acid (0.5 mL, 30 % v/v, 7 °C). The precipitate was separated by centrifugation (8000×g, 10 min, 21 °C). Afterwards, the absorbance of a supernatant sample was measured at a wavelength of 410 nm (DU-650, Beckman). The unit of proline peptidase activity was expressed as 1 nmol p-nitroaniline (pNa) released within 1 min at 30 °C and at pH 7.0.
Determination of the transglutaminase activity
The activity of TG was determined using an assay kit (CS1070, Sigma-Aldrich, St. Louis, MO, USA). The assay is based on TG catalysis of a covalent bond formation between a free amine group of poly-l-lysine, which is covalently attached to the plate surface, and the γ-carboxamide group of biotin-TVQQEL-OH substrate. The amount of immobilised biotin is proportional to the amount of active TG in the sample. The amount of immobilised biotin was determined using streptavidin–peroxidase and TMB substrate. The unit of transglutaminase activity was expressed as 1 nmol of TMB degraded within 1 min at 30 °C.
Degree of protein hydrolysis and average peptide chain length
The degrees of hydrolysis (DH) of wheat proteins were measured by the o-phthaldialdehyde (OPA) method. Native and modified samples of flour (100 mg) were suspended in borate buffer (1 mL, 12.5 mmol/L, pH 8.5, Na2B4O7·10H2O, H3BO3) with SDS (2 % w/v) mixed on magnetic stirrer (30 min, 21 °C, IKA-Combimag, Werke GmbH & Co. KG, Staufen, Germany) and centrifuged (5000×g, 10 min, 21 °C). Supernatants (125 μL) were mixed with the OPA reagent (5 mL). The OPA reagent was composed of o-phthaldialdehyde (0.08 % w/v), methanol (2 % v/v), 2-mercaptoethanol (0.2 % v/v) filled up to 1 L with borax solution (0.1 mol/L, Na2B4O7·10H2O). The mixture was allowed to stand for 20 min before measurement of the absorbance at a wavelength of 340 nm (DU-650, Beckman). The number of amino groups was determined with reference to the l-leucine standard curve between 1.25 × 10−6 and 1.0 × 10−5 mmol/L. The increase in α-amino groups between flour proteins and hydrolysates was attributed to proteolysis and degree of hydrolysis (DH) was calculated by the following equation:
where n T was the total number of amino groups in native flour after acid hydrolysis, n i was the number of amino groups in native flour, and α was the number of free amino groups in the modified flour.
The total number of amino groups in flour was determined after acid hydrolysis. The samples of flour (100 mg) were suspended in HCl solution (5 mL, 6 mol/L), sealed in glass ampule and then incubated (24 h, 105 °C). Hydrolysed flour was neutralised with NaOH solution (5 mL, 6 mol/L) and centrifuged (5000×g, 10 min, 21 °C). The total number of amino groups in flour was determined by the OPA method.
Average peptide chain length (APCL) was determined both before and after acid hydrolysis. Samples of flour (100 mg) were suspended in HCl solution (5 mL, 6 mol/L), sealed in glass ampule and then incubated (24 h, 105 °C). Hydrolysed flour was neutralised with NaOH solution (5 mL, 6 mol/L) and centrifuged (5000×g, 10 min, 21 °C). The number of amino groups in flour was determined by the OPA method. The average chain length was obtained from the following formula and expressed as amino acid units (AAU):
where n AH was the number of amino groups in modified flour after acid hydrolysis, α was the number of free amino groups in the modified flour.
Electrophoretic characterisation of wheat flour proteins
NuPage electrophoresis
The electrophoretic analyses of the samples of protein from native and modified wheat flour were performed by the NuPAGE method, in the NuPAGE Novex Bis–Tris 4-12 % gel, using the NuPAGE MES-SDS buffer and the XCell SureLock Mini-Cell electrophoresis system. All reagents for electrophoresis were purchased from Life Technologies (Warsaw, Poland). The molecular weight standard (3.5–260 kDa, Novex Sharp Unstained Protein) was used. Samples of the quantity of 10 μL were loaded on the gel from mixture of the following composition: 10 μL the protein sample (100 μg/mL); 2.5 μL NuPAGE LDS sample buffer; 1 μL NuPAGE reducing agent; 6.5 μL water. The samples were heated for 10 min at 70 °C. The separation was performed at the voltage of 200 V, with initial current intensity of 100–115 mA, for 40 min. Then the gels were stained using the SimpleBlue SafeStain, according to the producer’s procedure. The qualitative and relative-quantitate analysis of the electropherograms was performed using Gel Logic 200 system (Eastman Kodak Company, New York, USA) and Molecular Imaging Software (ver. 4.0, Eastman Kodak Company).
Free zone capillary electrophoresis FZCE
In the sequential extraction, native and modified wheat flour (60 mg) was extracted by stirring (30 min, 21 °C) with phosphate buffer (2 mL, 66 mmol/L, pH 7.6), centrifuged (5000×g, 15 min, 21 °C). The supernatant, containing the albumins and globulins, was discarded. The pellet was again extracted by stirring and centrifuged with the same conditions. Next, the pellet was washed by stirring (15 min, 21 °C) with 1 mL of deionised water and centrifuged as above. The supernatant was discarded, and the pellet was extracted by stirring (30 min, 21 °C) with aqueous ethanol (1 mL, 60 % v/v) and centrifuged as above. The pooled supernatant, mainly gliadins, was used for the FZCE analysis.
FZCE was carried out with a capillary electrophoresis system (BioFocus 3000, BioRad Laboratories, Hercules, CA, USA) equipped with a UV–visible detector. The electrophoretic separation was conducted with the use of a silica capillary with a diameter of 50 μm and effective length of 24 cm. Before each separation, the capillary was stabilised by the application of the following three-stage rinsing: H2PO4 (0.1 mol/L, 600 s); deionised water (120 s); separation phosphate-glycine buffer (100 mmol/L, pH 2.5, 900 s) with acetonitrile (20 % v/v) and (hydroxypropyl)methyl cellulose (0.05 % w/v). A sample of gliadin was pressure injected onto the capillary (5 psi·s) and separated from + to −, under an applied voltage of 20 kV, at a temperature of 25 °C, for 25 min. The qualitative composition of gliadin was analysed at a wavelength of 200 nm using a UV–visible detector, based on the time of peak migration, by means of BioFocus Integrator software (BioRad). The relative content of gliadins was measured as area (AU·s) under the electropherograms and calculated as percentage of gliadins in non-treated wheat flour.
Protein immunoreactivity
The native and modified wheat flour (250 mg) was extracted by stirring (30 min, 21 °C) with aqueous ethanol (1 mL, 60 % v/v) and centrifuged (5000×g, 10 min, 21 °C). The supernatant, containing alcohol-soluble proteins, was analysed using Ridascreen Gliadin Competitive kit (R7021, R-Biopharm AG, Darmstadt, Germany) with R5 monoclonal antibody recognises potentially toxic sequence QQPFP.
Immunoreactivity level of enzyme-treated proteins was estimated in the form of two indicators: effective immunoreactivity (EI) and relative residual immunoreactivity (RRI). EI was obtained from the following formula:
where C was content of QQPFP toxic peptide and α was the number of free amino groups in the enzymatically modified flour.
RRI was obtained from the following formula:
where EIm was effective immunoreactivity of the enzymatically modified flour proteins, EI0 was effective immunoreactivity of the native wheat flour proteins.
Statistical analysis
Results presented are the mean values ± standard deviation of three assays from two independent experiments. Data were compared by ANOVA and Bonferroni test. The statistical significance (p < 0.05) was determined by using Statistica software (ver 10. StatSoft, Tulusa, OK, USA).
Results
Activity of enzymatic preparations
In the presented experiments, enzymes hydrolysing proteins and peptides including: PEP synthesised by A. niger and peptidases LA and LS synthesised by L. acidophilus 5e2 and L. sanfranciscensis DSM20663, as well as a protein cross-linking enzyme TG were used to modify wheat proteins. Proline-specific peptidases synthesised by L. acidophilus 5e2 were characterised by Brzozowski et al. [14]. This strain synthesises intracellular prolyl endopeptidase, X-prolyl dipeptidyl aminopeptidase and proline iminopeptidase, similarly to L. plantarum, L. helveticus, L. casei and L. paracasei [31, 32]. Cell homogenate of L. sanfranciscensis DSM20663 also exhibits activity of proline-specific peptidases. The presence of intracellular peptidases was also found in cells of lactobacilli isolated from baker’s sourdoughs. L. sanfranciscensis CB1 synthesises metallodipeptidases releasing only hydrophobic amino acid-containing dipeptides, aminopeptidases of broad substrate specificity and X-prolyl dipeptidyl aminopeptidase hydrolysing X-P dipeptides from the N-terminus of the polypeptide chains [32, 33].
L. sanfranciscensis DSM20663 synthesises proteolytic enzymes characterised by the significantly (p < 0.05, ANOVA, Bonferroni test) higher endopeptidase activity of 4.1 ± 0.1 U/mg, compared to the L. acidophilus 5e2 strain (Table 1). The crude preparation of intracellular proteolytic enzymes from L. sanfranciscensis DSM20663 exhibits a lower activity of proline-specific peptidases than the preparation obtained from L. acidophilus 5e2. On the other hand, the enzyme preparation derived from A. niger demonstrates the highest activity of prolyl endopeptidase of 161.6 ± 0.7 U/mg. In contrast, the activity of PEP synthesised by L. acidophilus 5e2 and L. sanfranciscensis DSM20663 equals 12.7 ± 0.2 and 4.2 ± 0.7 U/mg, respectively.
Modification of wheat proteins using individual enzymes
Wheat grain proteins were modified with an addition of single-enzyme preparations including: TG, PEP, LA and LS. Native, non-modified flour was characterised by a content of α-amino groups of 1.85 ± 0.01 μmol/mL, and average polypeptide chain length of 32.0 ± 0.2 AAU (Fig. 1). The ingredients of flour were reacted with TG in a reaction medium of pH 5.0, pH 6.0, pH 7.0 and pH 8.5. After the reaction with TG under acidic conditions (pH 5.0), no significant changes in the contents of α-amino groups were observed, compared to native flour. However, the average polypeptide chain length slightly decreased to 31.4 ± 0.1 AAU. With the increase in alkalinity of the reaction medium, the content of α-amino groups in the samples decreased, which resulted in a decrease in the degree of protein hydrolysis. In the alkaline medium (pH 8.5), it turned negative (−1.22 ± 0.01 %), which confirms cross-linking properties of TG. The average polypeptide chain length increased from 32.0 ± 0.1 AAU for the native flour to 52.7 ± 0.1 AAU for the flour modified with TG (pH 8.5). However, in the reaction media of pH 6.0 and pH 7.0, the determined degree of protein hydrolysis did not differ significantly (p < 0.05, ANOVA, Bonferroni test) in comparison with the proteins of the native flour. The average polypeptide chain length of the proteins of the TG-modified flour in a medium of pH 7.0 was significantly greater than in the control sample.
Content of α-amino groups (AAG), degree of protein hydrolysis (DH) and average peptide chain length (APCL) of native wheat flour samples and treated with transglutaminase (TG) at different acidity and treated with proteolytic enzymes: prolyl endopeptidase (PEP), peptidases from L. acidophilus 5e2 (LA) and L. sanfranciscensis DSM20663 (LS) at 30 °C during 3 h. The different letters above the bar presented group of data for AAG, DH and APLC which are significantly different (p < 0.05, ANOVA, Bonferroni test)
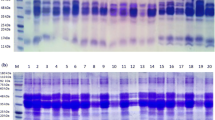
The conducted experiments demonstrate that under alkaline conditions the cross-linking bonds are formed in proteins, which is confirmed by the separation of proteins using the NuPAGE method (Fig. 2). Transamidation of wheat proteins results in the formation of sparingly soluble high molecular weight aggregates, which do not show the electrophoretic mobility and remain in the wells of a gel as shown by Bauer et al. [34] experiments. With the increase in pH-value of the medium, the content of polymerisable protein fractions increases. Samples of proteins modified at pH 5.0 and pH 6.0 with the participation of TG of microbial origin demonstrate a similar fractional composition as the native sample (Fig. 2). On the other hand, in a neutral medium, the changes in the protein composition as a result of their cross-linking in the ranges of molecular weight of 67–88 kDa and 39–55 kDa, characteristic of high molecular weight glutenins (HMW-glutenins) and ω-gliadins, respectively, were reported. Modification of wheat flour conducted at pH 8.5 with TG demonstrated that also αβ-, γ-gliadins and low molecular weight glutenins (LMW-glutenins) undergo cross-linking, which was observed as a change in the intensity of polypeptide staining within the molecular weight range from 28 to 39 kDa.
Capillary electrophoresis of gliadins also revealed changes in these proteins after the reaction with TG in media of different pH. The content of isolated gliadins was statistically significantly lower (p < 0.05, ANOVA, Bonferroni test) than in the native sample, regardless of the acidity of the reaction medium. This means that cross-linking of gliadins occurs also under acidic conditions. Their content was reduced to a level of 75 ± 0.5 and 60.8 ± 0.5 % for the media with acidity of pH 5 and pH 6, respectively (Fig. 3). However, the least content of gliadins of 41.3 ± 0.4 % was observed in a sample modified with TG at pH 8.5. Analysis of fractional composition of gliadins demonstrated that decreasing acidity of reaction medium is associated with more effective cross-linking of ω-gliadins. Under acidic conditions (pH 5.0) 22 % of this prolamin fraction underwent polymerisation, while under alkaline conditions it was 89.6 %. The remaining fractions of wheat prolamins also form high molecular weight protein aggregates. The amount of αβ-, γ-gliadins in the medium of pH 8.5 was reduced by 52.3 and 56.1 %, respectively.
Relative content of gliadins and composition of native wheat flour samples and treated with transglutaminase (TG) at different acidity and treated with proteolytic enzymes: prolyl endopeptidase (PEP), peptidases from L. acidophilus 5e2 (LA) and L. sanfranciscensis DSM20663 (LS) at 30 °C during 3 h. The different letters on the bar presented group of data for αβ-, γ- and ω-gliadins which are significantly different (p < 0.05, ANOVA, Bonferroni test)
The flour hydrolysed using PEP, LA and LS was characterised by a statistically significantly (p < 0.05, ANOVA, Bonferroni test) higher content of free α-amino groups of respectively 2.84 ± 0.01, 9.06 ± 0.04 and 10.37 ± 0.06 μmol/mL, in comparison with the native flour (Fig. 1). The degree of protein hydrolysis for wheat degraded with LS (14.45 ± 0.11 %) and LA (12.23 ± 0.07 %) peptidases was significantly (p < 0.05, ANOVA, Bonferroni test) higher than in the sample of proteins subjected to the PEP-catalysed reaction (1.69 ± 0.01 %). The enzymatic breakdown of proteins resulted also in a decrease in the average polypeptide chain length from 32 ± 0.2 AAU for the native flour to 20.8 ± 0.1, 6.5 ± 0.0 and 5.7 ± 0.1 AAU for the flour hydrolysed with PEP, LA and LS, respectively.
The usefulness of preparations of LA and LS peptidases and PEP endopeptidase in the degradation of wheat storage proteins was confirmed by their electropherograms (Fig. 4). Changes in the intensity of polypeptide staining within molecular weight ranges of 67–88 kDa and 39–55 kDa indicate that all the applied proteolytic enzymes effectively degrade HMW-glutenins and ω-gliadins. Compared to the samples of native proteins, the hydrolysates obtained by reaction (3 h, 30 °C) with LS, LA and PEP were characterised by a content of ω5-gliadins lowered by 55.5, 30.8 and 23.6 %, respectively. In turn, the content of HMW-glutenins decreased by 62.4, 31.4 and 14.8 % as a result of the degradation of proteins using LS, LA and PEP, respectively. Changes in the quantitative composition of protein hydrolysates reacted (3 h, 30 °C) with LS were also observed in the molecular weight range of 28–39 kDa, characteristic for αβ-, γ-gliadins and LMW-glutenins. Enzymatic degradation of the proteins was also confirmed using capillary electrophoresis of gliadins (Fig. 3). In all the protein hydrolysates, statistically significant (p < 0.05, ANOVA, Bonferroni test) lower levels of wheat prolamins were recorded, in comparison with the native sample. Protein hydrolysates subjected to reactions with PEP, LA and LS contained 18.6, 35.3 and 38.6 % less gliadins, respectively, than the non-modified sample. It was confirmed that among the analysed fractions ω-gliadins are degraded to the greatest extent as a result of LS and LA peptidase activity. Among the investigated preparations of proteolytic enzymes, PEP endopeptidase hydrolysed gliadins least effectively, reducing the share of individual αβ, γ and ω fractions by 14.9, 11.1 and 47.8 %, respectively.
Nu-Page analysis of reduced samples of wheat flour proteins after hydrolysis (1.5 h) with peptidases from L. sanfranciscensis DSM20663 (line 2), prolyl endopeptidase (line 3), peptidases from L. acidophilus 5e2 (line 4), following cross-linking (1.5 h) with transglutaminase and wheat flour samples cross-linked (1.5 h) with transglutaminase following hydrolysis (1.5 h) with peptidases from L. sanfranciscensis DSM20663 (line 5), prolyl endopeptidase (line 6), peptidases from L. acidophilus 5e2 (line 7) and wheat flour samples hydrolysed by peptidases from L. sanfranciscensis DSM20663 (line 8), prolyl endopeptidase (line 9), peptidases from L. acidophilus 5e2 (line 10) at 30 °C. Line 1 is the molecular weights marker
The non-modified wheat flour was characterised by the content of immunoreactive peptide with the QQPFP sequence at a level of 30.7 ± 0.2 mg/g (Table 2). As a result of TG-catalysed reaction, the content of the peptide detected in polypeptide sequences decreased under neutral and alkaline conditions and equalled 15.7 ± 0.0 and 3.6 ± 0.2 mg/g, respectively. However, in the acidic medium at pH 6.0 and pH 5.0, the content of detected QQPFP sequences was statistically higher (p < 0.05, ANOVA, Bonferroni test) in comparison with the native sample and equalled 32.7 ± 0.2 and 43.4 ± 0.5 mg/g, respectively. The effectiveness of protein detoxification is evidenced by the content of detected toxic QQPFP sequences relative to the content of free α-amino groups. The experiments showed that TG under alkaline and neutral conditions maximally reduces the EI value from 16.7 ± 0.6 mg/μmol for the native sample to 3.3 ± 0.2 and 8.8 ± 0.0 mg/μmol, respectively. Furthermore, the RRI for TG-modified proteins at pH 8.5 had a low value of 19.7 ± 1.2 %. Under acidic conditions, it equalled 139.2 ± 1.8 and 106.6 ± 0.6 % for the protein reacted with TG, at pH 5.0 and pH 6.0, respectively.
Hydrolysates of wheat flour proteins were characterised by a decreased immunoreactivity of 63.8 ± 1.8, 53.4 ± 2.6 and 55.2 ± 1.9 %, after the reaction with PEP, LA and LS, respectively. The lowest contents of the immunoreactive QQPFP peptide of 16.4 ± 0.8 and 16.9 ± 1.1 mg/g were observed for proteins degraded with LA and LS, respectively. However, in the samples hydrolysed with PEP, the content of determined toxic QQPFP sequences was statistically significantly higher (p < 0.05, ANOVA, Bonferroni test) and equalled 19.6 ± 0.5 mg/g. The lowest EI values of 1.8 ± 0.1 and 1.6 ± 0.1 mg/μmol were also determined for the hydrolysates obtained by reaction with LA and LS peptidase, respectively. The EI of the proteins degraded by PEP was statistically significantly higher (p < 0.05, ANOVA, Bonferroni test) in comparison with other hydrolysates and equalled 6.9 ± 0.2 mg/μmol. Also RRI values for the samples of proteins subjected to reactions catalysed by LA and LS peptidases were statistically significantly (p < 0.05, ANOVA, Bonferroni test) lower than for the samples of hydrolysates of proteins modified with the addition of PEP.
Modification of wheat proteins using two enzymes
Wheat proteins were also modified using two enzymes. In the first experimental system, the hydrolysis of cross-linked proteins was performed by the introduction of TG, and subsequently after 90 min of proteolytic enzymes into the reaction medium. In the second experimental system, the cross-linking of hydrolysates was performed by the addition of proteolytic enzymes, and subsequently after 90 min of TG to wheat flour proteins.
Pre-crosslinked and then hydrolysed wheat proteins were characterised by statistically significantly (p < 0.05, ANOVA, Bonferroni test) higher content of free α-amino groups than the native flour. Their content was 1.98 ± 0.01, 5.24 ± 0.07 and 5.98 ± 0.03 μmol/mL for TG-PEP, TG-LA and TG-LS preparations, respectively (Fig. 5). Changing the order of the processes or pre-hydrolysis of proteins followed by their cross-linking contributed to the increased content of free α-amino groups. Proteins modified with two enzymes were characterised by a statistically significantly lower (p < 0.05, ANOVA, Bonferroni test) degree of hydrolysis in comparison with the hydrolysates obtained as a result of LA and LS peptidase activity. The highest degree of hydrolysis of proteins modified in a two-enzyme system was determined for cross-linked hydrolysates obtained by reaction with LA-TG (8.84 ± 0.03 %) and LS-TG (11.69 ± 0.02 %).
Content of α-amino groups (AAG), degree of protein hydrolysis (DH) and average peptide chain length (APCL) of native wheat flour sample and wheat flour samples after hydrolysis (1.5 h) with prolyl endopeptidase (TG–PEP), peptidases from L. acidophilus 5e2 (TG-LA), L. sanfranciscensis DSM20663 (TG–LS) following cross-linking (1.5 h) with transglutaminase and wheat flour samples cross-linked (1.5 h) with transglutaminase following hydrolysis (1.5 h) with prolyl endopeptidase (PEP–TG), peptidases from L. acidophilus 5e2 (LA-TG), L. sanfranciscensis DSM20663 (LS–TG) at 30 °C. The different letters above the bar presented group of data for AAG, DH and APLC which are significantly different (p < 0.05, ANOVA, Bonferroni test)
The reaction of cross-linking protein hydrolysates also favours the reduction in the average polypeptide chain length. The samples of hydrolysates obtained as a result of PEP, LA and LS activity and subjected to polymerisation with TG were characterised by the average polypeptide chain length of 27.1 ± 0.3, 8.4 ± 0.0 and 6.8 ± 0.0 AAU, respectively. On the other hand, protein aggregates subjected to hydrolysis with PEP, LA and LS were characterised by the average polypeptide chain length of 29.8 ± 1.1, 11.3 ± 0.1 and 9.9 ± 0.1 AAU, respectively.
NuPAGE electrophoresis confirmed the change in the composition of wheat flour proteins subjected to two-enzymatic modification. Both cross-linking of protein hydrolysates and hydrolysis of protein aggregates remove the fraction of ω5-gliadins from the study samples (Fig. 4). However, ω1,2-gliadins remain present in the reaction medium in all tested samples. Within the range of molecular weight of 67–88 kDa, the presence of a band corresponding to HMW-glutenins was observed only in the sample modified with TG-PEP enzymes. Changes in the staining intensity of polypeptides within the molecular weight range from 28 to 39 kDa indicate that two-enzymatic modification affects also αβ-, γ-gliadins and LMW-glutenins.
Changes in the composition of wheat prolamins were confirmed using capillary electrophoresis. Regardless of the applied enzyme combination, the content of gliadins in flour samples was statistically significantly lower (p < 0.05, ANOVA, Bonferroni test) than in the native sample and equalled from 60.2 ± 0.5 to 73.6 ± 0.4 % for the samples of the proteins modified with the addition of LS-TG and TG-PEP enzymes, respectively (Fig. 6). It has been shown that the use of LS-TG and TG-LS preparations for a two-enzymatic modification of proteins effectively reduces the content of ω-gliadins from the initial 14.4 ± 0.3 to values of 8.6 ± 0.2 and 8.9 ± 0.3 %, respectively. In contrast, the αβ-gliadin fraction is the most resistant to a two-step treatment with TG and proteolytic enzymes. Its content in the total content of gliadins is reduced by 23.4, 34.9 and 35.1 % for TG–PEP, TG–LA and TG–LS enzymatic systems, respectively. It should be noted that the cross-linking of protein hydrolysates reduces the content of individual gliadin fractions in wheat grain proteins more effectively that the degradation of protein aggregates.
Relative content of gliadins and composition of native wheat flour sample and wheat flour samples after hydrolysis (1.5 h) with prolyl endopeptidase (TG–PEP), peptidases from L. acidophilus 5e2 (TG–LA), L. sanfranciscensis DSM20663 (TG–LS) following cross-linking (1.5 h) with transglutaminase and wheat flour samples cross-linked (1.5 h) with transglutaminase following hydrolysis (1.5 h) with prolyl endopeptidase (PEP–TG), peptidases from L. acidophilus 5e2 (LA–TG), L. sanfranciscensis DSM20663 (LS–TG) at 30 °C. The different letters on the bar presented group of data for αβ-, γ- and ω-gliadins which are significantly different (p < 0.05, ANOVA, Bonferroni test)
Immunological analysis of proteins subjected to two-step enzymatic modification showed a reduction in the content of toxic QQPFP sequence both in the samples of cross-linked protein hydrolysates, and in cross-linked proteins, as compared to the native sample. The lowest content of immunoreactive epitope which equalled 16.9 ± 0.4 mg/g was found in proteins modified with TG–LS (Table 2). However, this value is higher than for the sample of cross-linked proteins at pH 8.5, for which a value of 3.6 ± 0.2 mg/g of the peptide was recorded. Cross-linking of protein hydrolysates caused a decrease in the immunoreactivity of samples to values of 83.4 ± 3.1 and 87.5 ± 3.2 % for PEP–TG and LA–TG preparations, respectively. In turn, cross-linked proteins obtained as a result of a reaction with LS–TG were characterised by immunoreactivity which was not statistically significantly different (p < 0.05, ANOVA, Bonferroni test) from the native sample. The determined value of EI and RRI for all samples of proteins modified in a two-step reaction was statistically significantly lower (p < 0.05, ANOVA, Bonferroni test) than for the native sample. Wheat proteins undergo the most effective modifications favouring the reduction in immunoreactivity, when TG–LS and LS–TG enzymatic combinations are used. The EI values for protein samples modified with TG–LS and LS–TG equal 2.8 ± 0.1 and 3.4 ± 0.1 mg/μmol, respectively. Small EI values suggest that polypeptides and peptides are modified within the QQPFP epitope detected by the R5 antibody. It should be noted that the hydrolysis of pre-crosslinked proteins favours more the reduction in protein immunoreactivity than their cross-linking.
Discussion
Lactic acid bacteria require for their growth the presence of nitrogen sources in the form of peptides and amino acids in the culture medium. Cereal proteins contain large amounts of these compounds and are characterised by high endogenous peptidases activity. The activity of native peptidases results in the release of low molecular weight compounds being a nitrogen source for growing lactic acid bacteria from grain storage proteins [35]. The growth of LAB on substrates rich in peptides reduces the activity of cell wall-associated proteins [36]. In some lactic acid bacteria isolated from baker’s sourdoughs, including L. sanfranciscensis ATCC 27651T and L. sanfranciscensis DSM 20451T, no extracellular peptidase activity was found [35, 37]. However, lactic acid bacteria synthesise three intracellular endopeptidases distinguished based on their substrate preferences (PepO—oligoendopeptidase, PepF—endopeptidase cutting peptide bonds between F and S, PepE—general endopeptidase), four aminopeptidases (PepN and PepC—general aminopeptidases, PepA—narrow specificity aminopeptidase releasing E or D, PepL—leucyl aminopeptidase), tripeptidase (PepT—general tripeptidase) and dipeptidase (PepV—general dipeptidase) [38]. In turn, hydrolysis of peptide bonds involving proline requires the synthesis of proline-specific peptidases (PepX—X-prolyl dipeptidyl aminopeptidase, PepQ—prolidase, PepR—prolinase, PepI—proline iminopeptidase) [36].
In the degradation of gluten proteins also the enzymes of physiologically active lactic acid bacteria can be employed. However, due to low activity of peptidases produced by microorganisms hydrolysis of proteins may take up to 24–48 h [19, 21]. Therefore, the pre-hydrolysis of cereal prolamins is conducted using endogenous grain enzymes or by the addition of fungal peptidases and then the released peptides are further degraded by lactic acid bacteria [22, 39].
In the present study, the use of the addition of the preparation of intracellular peptidases which are synthesised by lactic acid bacteria for the hydrolysis of wheat flour is suggested, which eliminates the need of waiting for the growth of microbial cells. The use of the preparation of peptidases synthesised by L. sanfranciscensis DSM20663 and L. acidophilus 5e2 allows for a more intense hydrolysis of proteins, as compared to the use of PEP synthesised by A. niger. This results from the fact that LAB synthesise a mixture of peptidases with a broad spectrum of activity and different specificity for amino acid residues forming peptide bonds in the polypeptide chain [21, 30]. However, the number of bonds degraded by PEP is limited to those formed by at least one proline residue [25].
Differentiated degradation of prolamin fraction by PEP, results from various contents of proline residues. The content of this amino acid in αβ, γ and ω gliadins is 16, 17 and 23 mol %, respectively. In addition, the presence of cysteine in the C-terminal domain of αβ- and γ-gliadins allows for the formation of three and four intramolecular disulphide bonds, respectively [40], which may reduce the availability of peptide bonds formed by proline. In contrast, ω-gliadin hardly contains cysteine residues and thus does not participate in the formation of disulphide bonds. In addition, the central domain of ω-gliadin contains a repeated motif from 6 to 11 amino acids of the PFPQ(Q)(Q)PQ(Q)(Q)(Q) sequence, comprising proline residues [41]. In turn, Wieser and Koehler [42] report that the central region of ω5-gliadin and ω1,2-gliadin is characterised by the presence of motifs with (Q)QQQFP sequence repeated 65 times and (QP)QQPFP sequence repeated 42 times, respectively. On the other hand, the central domain of γ-gliadin contains 15–16 times repeated motifs with the amount of residues from 8 to 12 and PFPQQ(Q)PQQ(PQQ) [41], (Q)QPQQPFP [42] or PQQPFPQ [43] amino acid sequences. The central domain of αβ-gliadins contains even less proline residues and is built of a fivefold repeated motif containing from 5 to 8 amino acids with a QPQPFPPQQPYP [42], P(F/Y)PQQQ(Q)(Q) [41], PQPQPFP or PQQPY [43] sequence. The content of proline residues enables the arrangement of gliadins from αβ fraction which is the least susceptible to PEP, followed by γ fraction to ω fraction which is degraded by PEP to the greatest extent.
The present experiments demonstrated that the addition of individual preparations of intracellular enzymes synthesised by L. acidophilus 5e2 and L. sanfranciscensis DSM20663 and PEP in an amount of 10U per gram of flour lowers its immunoreactivity by at least 35 % within 3 h. In the experiments, R5 monoclonal antibody was used which detect among others QQPFP, LQLQPFP, QLPYP, PQPF and PQPFP amino acid sequences present in the primary structure of gliadins. The efficiency of degradation of coeliac toxic fragments of peptides was confirmed using capillary electrophoresis. The change in gliadin content in samples hydrolysed with LA, LS and PEP was approx. 35, 40 and 20 %, respectively, as compared to the native sample. The present experiments are confirmed by the study of Gerez et al. [44] who demonstrated that the mixture (at a ratio of 1:1) of cytoplasmic fractions of L. plantarum CRL775 and Pediococcus pentosaceus CRL 792 reduce the content of gliadins from approx. 94,000 mg/kg to approx. 41,000 mg/kg within 4 h. In turn, the use of a mixture of L. sanfranciscensis (LS3, LS10, LS19, LS23, LS38 and LS47) strains reduces the content of gliadins in sourdough by 27,000 mg/kg within 48 h of incubation [19].
The possibility of degradation of coeliac toxic wheat proteins using lactic acid bacteria was also confirmed by Di Cagno et al. [21]. Bacteria isolated from sourdough including: L. alimentarius 15 M, L. brevis 14G, L. sanfranciscensis 7A and L. hilgardii 51B hydrolyse proline-rich peptides, including a 33-mer peptide resistant to digestion with the enzymes of the human gastrointestinal system, which comprises coeliac toxic sequences of amino acids. Similar characteristics were demonstrated for the cytoplasmic enzyme fraction synthesised by these bacteria. The addition of cytoplasmic fraction of bacteria to sourdough produced from a mixture of wheat, oat, millet and buckwheat flour (at a ratio of 3:1:4:2) with the participation of LAB causes almost complete degradation of gliadins and alcohol-soluble low molecular weight peptides, within 24 h. The degrees of gluten polypeptide hydrolysis were form 50.0 to 98.7 % and probably were still by far too high to make the products safe for coeliac people. However, the bread produced of such sourdough did not cause an increase in the intestinal permeability in 13 out of 17 patients with diagnosed coeliac disease.
The peptidases isolated from LAB and/or sourdough microflora are not able to degrade gluten proteins to produce products suitable for coeliac people. Rizzello et al. [19] proposed using combination of the activity of LAB and fungal peptidases to degrade and detoxify of gluten proteins. The production of sourdough by LAB and fungal peptidases reduces immunoreactivity of gliadins and glutenins.
In a modification of wheat proteins, TG, which belongs to the acyltransferase group and catalyses the transfer reaction of acyl group between γ-carboxamide group of glutamine and different primary amino groups of various compounds, including proteins, is also used. This enzyme can catalyse deamidation by transferring the acyl group of glutamine to a molecule of water with the formation of glutamic acid and ammonia. In turn, the cross-linking of different proteins is a result of transamidation, or a formation of a covalent bond ε-(γ-Q)-K between the ε-amino group of lysine in one protein and γ-carboxamide group of glutamine in another protein [45, 46]. TG used in bread technology is responsible for the formation of large insoluble polymers and allows for a modification of the structure of crumb, especially in case of bread produced of poor quality flour or flour containing proteins degraded by insect peptidases [47, 48]. Under acidic conditions, TG primarily catalyses reactions of glutamine deamination to glutamic acid [7]. On the other hand, under alkaline conditions, transamidation reactions dominate and bonds between polypeptide chains are formed [28]. The present experiments also demonstrated the possibility of protein cross-linking by TG under alkaline conditions. With the decrease in the acidity of the reaction medium, the average polypeptide chain length increases which indicates the formation of cross-links between polypeptides and the structures of aggregated proteins. Tissue transglutaminase synthesised in the human body has similar properties, and it increases the degree of cross-linking of α2(56-68)-gliadin peptide with collagen with a decrease of acidity from pH 6.0 to pH 7.5 [49]. Modifications of proteins using TG conducted under acidic conditions revealed small changes in average polypeptide chain length, but their immunoreactivity increased from 6.6 to 41.4 %, as compared to the native sample. These observations suggest that under acidic conditions TG catalyses mostly deamidation reactions. Similar properties of TG were observed by Fleckenstein et al. [50]. In turn, Kanerva et al. [51] demonstrated that chemical deamidation (0.1 mol/L, 100 °C, 2 h) of gluten proteins decreased the affinity of antibody to peptides. This process abolished the recognition of deamidated gluten peptides by omega-gliadin and G12 antibody and decrease the recognition by R5 antibody. Authors, as opposed to presented results, used in experiments vital wheat gluten and synthetic peptides being toxic in coeliac disease. The differences in immunoreactivity suggested that the surroundings of gluten may be important to recognition of deamidated proteins.
Gliadins and glutenins due to the high content of glutamine residues, 35–56 and 36–38 mol %, respectively, are a very good substrate for the reaction with TG [40]. Unfortunately, the lysine content in wheat storage proteins is low and it equals 0.7 and 1.2 mol % for gliadins and glutenins, respectively. In turn, the concentration of this amino acid in whole grains and wheat flour is 2.8 and 2.2 %, respectively [52].
Modification of wheat flour using TG in a medium without acidity correction demonstrated that the cross-linking of the ω5 fraction of gliadins is the fastest. Other protein fractions also polymerise in the following order: ω1,2-, α- and γ-gliadins [34]. Different susceptibility of gliadins to the formation of high molecular weight protein aggregates may result from the different content of glutamine and lysine in their fractions. ω5-gliadin contains the largest content of glutamine residues, 56 mol %. On the other hand, ω1,2-, α- and γ-gliadins contain 44, 37 and 35 mol % of residues of glutamine, respectively, which is a donor of carboxamide groups in the cross-linking reaction [40]. The equally high content of glutamine in LMW and HMW glutenins of 38–42 and 36–37 mol %, respectively, promotes the formation of aggregate structures by these proteins [34]. At a large supply of glutamine residues, the content of lysine residues in proteins is a factor limiting the formation of isopeptide bonds. In gliadins its concentration is maximally 0.6, 0.9 and 0.6 mol % for α, γ and ω fractions, respectively. In turn, the content of lysine in LMW and HMW glutenins is 0.6 and 1.1 mol %, respectively [34, 53]. Typically, γ-gliadins contain two lysine residues, while α-gliadins and LMW-glutenins contain one residue of this amino acid. Its position in the polypeptide chain conditioning the susceptibility to the reaction with TG is essential. In LMW-glutenins lysine is located in the C-terminal domain next to the cysteine residue, which forms intramolecular disulphide bonds and blocks the access of TG to lysine. In turn, in α-gliadin lysine is situated in easily accessible N-terminal domain or in a region rich in glutamine residues, which promotes a cross-linking reaction of proteins [54]. Under the conditions of low lysine content or its absence, TG will catalyse deamidation reactions of carboxamide groups in glutamine residues to produce glutamic acid, which increases the immunoreactivity of peptides by an increase in their affinity to the antigens of the major histocompatibility complex MHC II (HLA-DQ2) [7].
The accessibility of amino acids in wheat protein samples can be increased by their pre-hydrolysis. The degradation of polypeptides by LA and LS peptidases releases peptides that are subsequently substrates for the reaction with TG. The proposed cross-linking process reduced the immunoreactivity of wheat proteins. Cross-linked proteins obtained as a result of LS and then TG treatment were characterised by the highest immunoreactivity (97.6 ± 2.9 %). This results from a greater ability of LS preparation to degrade wheat proteins (DH 14.45 ± 0.11 %), in comparison with other preparations: PEP (DH 1.69 ± 0.01 %) and LA (12.23 ± 0.07 %). It should be noted that modifications of wheat flour were conducted in aqueous medium at an initial pH of 6.2. The present study demonstrated that under acidic conditions, TG catalyses mainly deamidation reactions. Therefore, the initial hydrolysis of proteins using intracellular peptidases and PEP produces various amounts of peptides which subsequently undergo cross-linking and/or deamidation. On the other hand, a lower immunoreactivity of pre-crosslinked protein hydrolysates in comparison with native proteins can be explained by initial polymerisation of peptides and formation of isopeptide bonds. Conformational changes of proteins hinder the access of proteolytic enzymes and antibody recognising QQPFP sequence to peptide bonds.
The study of Stamnaes et al. [6] demonstrated that concentrations of the enzyme as well as of donors and acceptors of acyl groups play a significant role in the type of TG-catalysed reaction. Low TG concentrations promote deamidation reactions. Furthermore, deamidation and transamidation of peptides are strongly determined by the protein primary structure, and especially the location of proline at the +2 position relative to glutamine. The presence of proline at the +2 position affects the affinity of TG to the substrate and a tendency of TG to transamidation relative to deamidation. Peptides which are rapidly cross-linked by TG can be subsequently deamidated using the same enzyme. TG can also catalyse hydrolysis reactions of isopeptide bonds previously formed between peptides, which will result in the formation of glutamic acid instead of glutamine. The presence of proline at the +2 position relative to glutamine favours peptidolytic activity of TG. Thus, the absence of +2 proline allows for a direct deamidation of glutamine in peptides, and its indirect presence through transamidation followed by peptide hydrolysis can also result in their deamidation.
The study R5 monoclonal antibody was detected among other QQPFP and QLPYP amino acid sequences, which contain proline at +2 position. Pre-hydrolysis of wheat proteins and release of peptides which are substrates in the reaction with TG can directly and indirectly affect deamidation of glutamine and thus increase immunoreactivity of the analysed proteins.
Glutamine at the first position plays a significant role in binding R5 antibody to QQPFP epitope. Its substitution with glutamic acid reduces the ability of binding epitope to antibody, whereas the same process conducted outside the QQPFP sequence does not affect the affinity of antibody. TG favours deamidation of glutamine at the first position of the QQPFP sequence, contributing to the reduction in the capacity to bind antibody. The amino acids which precede the detected epitope also play an important role. Deamidation of glutamine at 2 and 3 positions, in PEQPFP and QQEPFP amino acid sequences, respectively, reduces the affinity for antibody. In turn, the substitution of glutamine with its acid derivative at position 2 in the QEQPFP sequence increases the affinity compared to the QQPFP sequence [13]. Deamidation of peptides by TG reduces their affinity for antibody. However, peptides isolated from γ-gliadins and glutenins as a result of deamination with the participation of TG become immunodominant, strongly interacting with T cells, and are toxic for the patients with diagnosed coeliac disease [8, 9]. The increase in the toxicity of peptides as a result of TG activity may reduce their detection, which results from their lower affinity for R5 antibody [51].
According to the Codex Alimentarius, only foods not exceeding a level of 20 mg/kg of gluten could be labelled “gluten-free” products [55]. Codex had set also the level of gluten to the range of 20–100 mg/kg for “low gluten” products. Experiments presented in this study lowered gluten content from 61,400 to 7200 mg/kg using TG at alkaline pH. So, it is still too much to labelled this flour gluten-free. However, the modified flour is not the end product and should be considered as raw material with reduced content of gluten. Proposed methods of enzymatic reduction in gluten immunoreactivity could be used as additional step of flour modification in sourdough technology.
References
Di Sabatino A, Corazza GR (2009) Coeliac disease. Lancet 373:1480–1493
Gilissen LJWJ, van der Meer IM, Smulders MJM (2014) Reducing the incidence of allergy and intolerance to cereals. J Cereal Sci 59:337–353
Simpson DJ (2001) Proteolytic degradation of cereal prolamins—the problem with proline. Plant Sci 161(5):825–838
Hausch F, Shan L, Santiago NA, Gray GM, Khosla C (2002) Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol 283:996–1003
Shan L, Qiao SW, Arentz-Hansen H, Molberg Ø, Gray GM, Sollid LM, Khosla C (2005) Identification and analysis of multivalent proteolytically resistant peptides from gluten: implications for celiac sprue. J Proteome Res 4(5):1732–1741
Stamnaes J, Fleckenstein B, Sollid LM (2008) The propensity for deamidation and transamidation of peptides by transglutaminase 2 is dependent on substrate affinity and reaction conditions. Biochim Biophys Acta 1784(11):1804–1811
Dekking EHA, Van Veelena PA, de Rua A, Kooy-Winkelaara EMC, Gröneveld T, Nieuwenhuizen WF, Koning F (2008) Microbial transglutaminases generate T cell stimulatory epitopes involved in celiac disease. J Cereal Sci 47(2):339–346
Arentz-Hansen H, McAdam SN, Molberg Ø, Fleckenstein B, Lundin KE, Jørgensen TJ, Jung G, Roepstorff P, Sollid LM (2002) Celiac lesion T cells recognize epitopes that cluster in regions of gliadin rich in proline residues. Gastroenterology 123:803–809
Vader W, Kooy Y, van Veelen P, De Ru A, Harris D, Benckhuijsen W, Peña S, Mearin L, Drijfhout JW, Koning F (2002) The gluten response in children with in celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology 122:1729–1737
Ciccocioppo R, Di Sabatino A, Corazza GR (2005) The immune recognition of gluten in coeliac disease. Clin Exp Immunol 140:408–416
Fraser JS, Engel W, Ellis HJ, Moodie SJ, Pollock EL, Wieser H, Ciclitira PJ (2003) Coeliac disease: in vivo toxicity of the putative immunodominant epitope. Gut 52:1698–1702
Cornel HJ, Wills-Johnson G (2001) Structure-reactivity relationship in coeliac-toxic gliadin peptides. Amino Acids 21:243–253
Kahlenberg F, Sanchez D, Lachmann I, Tuckova L, Tlaskalova H, Méndez E, Mothes T (2006) Monoclonal antibody R5 for detection of putatively coeliac-toxic gliadin peptides. Eur Food Res Technol 222:78–82
Brzozowski B, Bednarski W, Dziuba B (2009) Functional properties of Lactobacillus acidophilus metabolites. J Sci Food Agric 89:2467–2476
Brzozowski B, Lewandowska M (2014) Prolyl endopeptidase—optimization of medium and culture conditions for enhanced production by Lactobacillus acidophilus. Electron J Biotech 17:204–210
Gass J, Bethune MT, Siegel M, Spencer A, Khosla C (2007) Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology 133(2):472–480
Gobbetti M, Rizzello CG, Di Cagno R, De Angelis M (2007) Sourdough lactobacilli and coeliac disease. Food Microbiol 24:187–196
Montserrat V, Bruins MJ, Edens L, Koning F (2015) Influence of dietary components on Aspergillus niger prolyl endoprotease mediated gluten degradation. Food Chem 174:440–445
Rizzello CG, De Angelis M, Di Cagno R, Camarca A, Silano M, Losito I, De Vincenzi M, De Bari MD, Palmisano F, Maurano F, Gianfrani C, Gobbetti M (2007) Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: new perspectives for celiac disease. Appl Environ Microbiol 73:4499–4507
Siegel M, Bethune MT, Gass J, Ehren J, Xia J, Johannsen A, Stuge TB, Gray GM, Lee PP, Khosla C (2006) Rational design of combination enzyme therapy for coeliac sprue. Chem Biol 13:649–658
Di Cagno R, De Angelis M, Auricchio S, Greco L, Clarke C, De Vincenzi M, Giovannini C, D’Archivio M, Landolfo F, Parrilli G, Minervini F, Arendt E, Gobbetti M (2004) Sourdough bread made from wheat and nontoxic flours and started with selected Lactobacilli is tolerated in celiac patients. Appl Environ Microbiol 70(2):1088–1096
Rizzello CG, Curiel JA, Nionelli L, Vincentini O, Di Cagno R, Silano M, Gobbetti M, Coda R (2014) Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiol 37:59–68
Tack GJ, van de Water JM, Kooy-Winkelaar EM, van Bergen J, Meijer GA, von Blomberg BM, Schreurs MW, Bruins MJ, Edens L, Mulder CJ, Koning F (2010) Can prolyl endoprotease enzyme treatment mitigate the toxic effect of gluten in coeliac patients? Gastroenterology 138(5):1–54
Shan L, Marti T, Sollid LM, Gray GM, Khosla C (2004) Comparative biochemical analysis of three bacterial prolyl endopeptidases: implications for coeliac sprue. Biochem J 383:311–318
Stepniak D, Spaenij-Dekking L, Mitea C, Moester M, de Ru A, Baak-Pablo R, van Veelen P, Edens L, Koning F (2006) Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. Am J Physiol Gastrointest Liver Physiol 291(4):G621–G629
De Angelis M, Cassone A, Rizzello CG, Gagliardi F, Minervini F, Calasso M, Di Cagno R, Francavilla R, Gobbetti M (2010) Mechanism of degradation of immunogenic gluten epitopes from Triticum turgidum L. var. durum by sourdough lactobacilli and fungal proteases. Appl Environ Microbiol 76:508–518
Gerrard JA, Sutton KH (2005) Addition of transglutaminase to cereal products may generate the epitope responsible for coeliac disease. Trends Food Sci Technol 16(11):510–512
Gianfrani C, Siciliano RA, Facchiano AM, Camarca A, Mazzeo MF, Costantini S, Salvati VM, Maurano F, Mazzarella G, Iaquinto G, Bergamo P, Rossi M (2007) Transamidation of wheat flour inhibits the response to gliadin of intestinal T cells in celiac disease. Gastroneterology 133:780–789
Majk I, Leszczyńska J, Łącka A (2011) Immunoreactivity of chemically cross-linked gluten and hydrolysates of wheat flour. Biotechnol Food Sci 75:27–34
Rollán G, De Angelis M, Gobbetti M, de Valdez GF (2005) Proteolytic activity and reduction of gliadin-like fractions by sourdough lactobacilli. J Appl Microbiol 99:1495–1502
Tobiassen RO, Stepaniak L, Sørhaug T (1997) Screening for differences in the proteolytic system of Lactococcus, Lactobacillus and Propionibacterium. Z Lebensm Unters Forsch A 204:273–278
Gallo G, De Angelis M, McSweeney PLH, Corbo MR, Gobbetti M (2005) Partial purification and characterization of an X-prolyl dipeptidyl aminopeptidase from Lactobacillus sanfranciscensis CB1. Food Chem 9:535–544
Gänzle MG, Loponen J, Gobbetti M (2008) Proteolysis in sourdough fermentations: mechanisms and potential for improved bread quality. Trends Food Sci Technol 19:513–521
Bauer N, Koehler P, Wieser H, Schieberle P (2003) Studies on effects of microbial transglutaminase on gluten proteins of wheat. I Biochemical analysis. Cereal Chem 80:781–786
Vermeulen N, Pavlovic M, Ehrmann MA, Gänzle MG, Vogel RF (2005) Functional characterization of the proteolytic system of Lactobacillus sanfranciscensis DSM 20451T during growth in sourdough. Appl Environ Microbiol 71:6260–6266
Guédon E, Renault P, Ehrlich SD, Delorme C (2001) Transcriptional pattern of genes coding for the proteolytic system of Lactococcus lactis and evidence for coordinated regulation of key enzymes by peptide supply. J Bacteriol 183:3614–3622
Pepe O, Villani F, Oliviero D, Greco T, Coppola S (2003) Effect of proteolytic starter cultures as leavening agents of pizza dough. Int J Food Microbiol 84:319–326
Christensen JE, Dudley EG, Pederson JA, Steele JL (1999) Peptidases and amino acid catabolism in lactic acid bacteria. Anton Leeuw 76:217–246
Loponen J, Sontag-Strohm T, Venäläinen J, Salovaara H (2007) Prolamin hydrolysis in wheat sourdoughs with differing proteolytic activities. J Agric Food Chem 55:978–984
Wieser H (2007) Chemistry of gluten proteins. Food Microbiol 24(2):115–119
Ang S, Kogulanathan J, Morris GA, Kök MS, Shewry PR, Tatham AS, Adams GG, Rowe AJ, Harding SE (2010) Structure and heterogeneity of gliadin: a hydrodynamic evaluation. Eur Biophys J 39(2):255–261
Wieser H, Koehler P (2008) The biochemical basis of celiac disease. Cereal Chem 85(1):1–13
Kączkowski J (2002) Nowe poglądy na strukturę i funkcje białek zapasowych zbóż na przykładzie pszenicy (Triticum aestivum L.). Biul IHAR 223/224:3–31
Gerez CL, Dallagnol A, Rollán G, Font de Valdez G (2012) A combination of two lactic acid bacteria improves the hydrolysis of gliadin during wheat dough fermentation. Food Microbiol 32(2):427–430
Kączkowski J (2005) Transglutaminase—an enzyme group of extended metabolic and application possibilities. Pol J Food Nutr Sci 14(55):3–12
Yokoyama K, Nio N, Kikuchi Y (2004) Properties and applications of microbial transglutaminase. Appl Microbiol Biotechnol 64:447–454
Larré C, Denery-Papini S, Popineau Y, Deshayes G, Desserme C, Lefebvre J (2000) Biochemical analysis and rheological properties of gluten modified by transglutaminase. Cereal Chem 77(2):121–127
Bonet A, Caballero PA, Gómez M, Rosell CM (2005) Microbial transglutaminase as a tool to restore the functionality of gluten from insect-damaged wheat. Cereal Chem 82(4):425–430
Dieterich W, Esslinger B, Trapp D, Hahn E, Huff T, Seilmeier W, Wieser H, Schuppan D (2006) Cross linking to tissue transglutaminase and collagen favours gliadin toxicity in coeliac disease. Gut 55:478–484
Fleckenstein B, Molberg Ø, Qiao SW, Schmid DG, von der Mülbe F, Elgstøen K, Jung G, Sollid LM (2002) Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process. J Biol Chem 277(37):34109–34116
Kanerva P, Brinck O, Sontag-Strohm T, Salovaara H, Loponen J (2011) Deamidation of gluten proteins and peptides decreases the antibody affinity in gluten analysis assays. J Cereal Sci 53:335–339
Shewry PR (2007) Improving the protein content and composition of cereal grain. J Cereal Sci 46:239–250
Gianibelli MC, Larroque OR, MacRitchie F, Wrigley CW (2001) Biochemical, genetic and molecular characterization of wheat endosperm proteins. Cereal Chem 78:635–646
Müller S, Wieser H (1997) The location of disulphide bonds in monomeric γ-type gliadins. J Cereal Sci 26:169–176
Codex Alimentarius Commission (2008) Codex Standard 118-1979 (revised 2008),Foods for special dietary use for persons intolerant to gluten. FAO/WHO, Rome
Acknowledgments
The author greatly acknowledge the financial support from the National Science Centre Poland, under Research Project No. N N312 170739.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Bartosz Brzozowski declares that he has no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brzozowski, B. Immunoreactivity of wheat proteins modified by hydrolysis and polymerisation. Eur Food Res Technol 242, 1025–1040 (2016). https://doi.org/10.1007/s00217-015-2608-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-015-2608-6