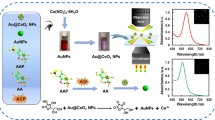
Abstract
Nanoceria have demonstrated a wide array of catalytic activity similar to natural enzymes, holding considerable significance in the colorimetric detection of alkaline phosphatase (ALP), which is a biomarker of various biological disorders. However, the issues of physiological stability and formation of protein corona, which are strongly related to their surface chemistry, limit their practical application. In this work, CeO2 nanoparticles characterized by enhanced dimensional uniformity and specific surface area were synthesized, followed by encapsulation with various polymers to further increase catalytic activity and physiological stability. Notably, the CeO2 nanoparticles encapsulated within each polymer exhibited improved catalytic characteristics, with PAA-capped CeO2 exhibiting the highest performance. We further demonstrated that the PAA-CeO2 obtained with enhanced catalytic activity was attributed to an increase in surface negative charge. PAA-CeO2 enabled the quantitative assessment of AA activity within a wide concentration range of 10 to 60 μM, with a detection limit of 0.111 μM. Similarly, it allowed for the evaluation of alkaline phosphatase activity throughout a broad range of 10 to 80 U/L, with a detection limit of 0.12 U/L. These detection limits provided adequate sensitivity for the practical detection of ALP in human serum.







Similar content being viewed by others
References
Chen ZJ, Wu HL, Shen YD, Wang H, Zhang YF, Hammock B, et al. Phosphate-triggered ratiometric fluoroimmunoassay based on nanobody-alkaline phosphatase fusion for sensitive detection of 1-naphthol for the exposure assessment of pesticide carbaryl. J Hazard Mater. 2022;424:127411.
Shaban SM, Jo SB, Hafez E, Cho JH, Kim DH. A comprehensive overview on alkaline phosphatase targeting and reporting assays. Coord Chem Rev. 2022;465:214567.
Ghosh SS, Wang J, Yannie PJ, Cooper RC, Sandhu YK, Kakiyama G, et al. Over-expression of intestinal alkaline phosphatase attenuates atherosclerosis. Circ Res. 2021;128(11):1646–59.
Peng C, Xing H, Xue Y, Wang J, Li J, Wang E. Ratiometric sensing of alkaline phosphatase based on the catalytical activity from Mn–Fe layered double hydroxide nanosheets. Nanoscale. 2020;12(3):2022–7.
Liu Y, Cavallaro PM, Kim B-M, Liu T, Wang H, Kühn F, et al. A role for intestinal alkaline phosphatase in preventing liver fibrosis. Theranostics. 2021;11(1):14.
Liu SG, Han L, Li N, Xiao N, Ju YJ, Li NB, et al. A fluorescence and colorimetric dual-mode assay of alkaline phosphatase activity via destroying oxidase-like CoOOH nanoflakes. J Mater Chem B. 2018;6(18):2843–50.
Han Y, Chen J, Li Z, Chen H, Qiu H. Recent progress and prospects of alkaline phosphatase biosensor based on fluorescence strategy. Biosens Bioelectron. 2020;148:111811.
Porat-Ophir C, Dergachev V, Belkin A, Vernick S, Freynd G, Katsnelson M, et al. Chip level agitation effects on the electrochemical sensing of alkaline-phosphatase expressed from integrated liver tissue. Sens Actuators, B Chem. 2015;213:465–73.
Yang R, Yan X, Li Y, Zhang X, Chen J. Nitrogen-doped porous carbon-ZnO nanopolyhedra derived from ZIF-8: new materials for photoelectrochemical biosensors. ACS Appl Mater Interfaces. 2017;9(49):42482–91.
Koncki R, Ogończyk D, Głąb S. Potentiometric assay for acid and alkaline phosphatase. Anal Chim Acta. 2005;538(1–2):257–61.
Sun D, Xu W, Liang C, Shi W, Xu S. Smart surface-enhanced resonance Raman scattering nanoprobe for monitoring cellular alkaline phosphatase activity during osteogenic differentiation. ACS Sensors. 2020;5(6):1758–67.
Zeng Y, Ren J-Q, Wang S-K, Mai J-M, Qu B, Zhang Y, et al. Rapid and reliable detection of alkaline phosphatase by a hot spots amplification strategy based on well-controlled assembly on single nanoparticle. ACS Appl Mater Interfaces. 2017;9(35):29547–53.
Ximenes VF, Campa A, Baader WJ, Catalani LH. Facile chemiluminescent method for alkaline phosphatase determination. Anal Chim Acta. 1999;402(1–2):99–104.
Liu X, Mei X, Yang J, Li Y. Hydrogel-involved colorimetric platforms based on layered double oxide nanozymes for point-of-care detection of liver-related biomarkers. ACS Appl Mater Interfaces. 2022;14(5):6985–93.
Zheng XT, Ananthanarayanan A, Luo KQ, Chen P. Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small. 2015;11(14):1620–36.
Mao G, Zhang Q, Yang Y, Ji X, He Z. Facile synthesis of stable CdTe/CdS QDs using dithiol as surface ligand for alkaline phosphatase detection based on inner filter effect. Anal Chim Acta. 2019;1047:208–13.
Liu P, Li D, Kang M, Pan Y, Wen Z, Zhang Z, et al. Emerging applications of aggregation‐induced emission luminogens in bacterial biofilm imaging and antibiofilm theranostics. Small structures. 2023;4(5).
Wang M, Liu H, Fan K. Signal amplification strategy design in nanozyme‐based biosensors for highly sensitive detection of trace biomarkers. Small Methods. 2023:2301049.
Ye K, Niu X, Song H, Wang L, Peng Y. Combining CeVO4 oxidase-mimetic catalysis with hexametaphosphate ion induced electrostatic aggregation for photometric sensing of alkaline phosphatase activity. Anal Chim Acta. 2020;1126:16–23.
Bornscheuer UT, Huisman G, Kazlauskas R, Lutz S, Moore J, Robins K. Engineering the third wave of biocatalysis. Nature. 2012;485(7397):185–94.
Breaker RR. DNA enzymes. Nat Biotechnol. 1997;15(5):427–31.
Meunier B, De Visser SP, Shaik S. Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chem Rev. 2004;104(9):3947–80.
Huang Y, Ren J, Qu X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev. 2019;119(6):4357–412.
Song H, Niu X, Ye K, Wang L, Xu Y, Peng Y. A novel alkaline phosphatase activity sensing strategy combining enhanced peroxidase-mimetic feature of sulfuration-engineered CoO x with electrostatic aggregation. Anal Bioanal Chem. 2020;412:5551–61.
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y, et al. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev. 2019;48(4):1004–76.
Wei H, Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42(14):6060–93.
Kim YG, Lee Y, Lee N, Soh M, Kim D, Hyeon T. Ceria‐based therapeutic antioxidants for biomedical applications. Advanced Mater. 2023:2210819.
Baldim V, Yadav N, Bia N, Graillot A, Loubat C, Singh S, et al. Polymer-coated cerium oxide nanoparticles as oxidoreductase-like catalysts. ACS Appl Mater Interfaces. 2020;12(37):42056–66.
Song H, Ye K, Peng Y, Wang L, Niu X. Facile colorimetric detection of alkaline phosphatase activity based on the target-induced valence state regulation of oxidase-mimicking Ce-based nanorods. J Mater Chem B. 2019;7(38):5834–41.
Li Y, Liu J. Nanozyme’s catching up: activity, specificity, reaction conditions and reaction types. Mater Horiz. 2021;8(2):336–50.
Asati A, Santra S, Kaittanis C, Nath S, Perez JM. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angewandte Chemie - Int Edition. 2009;121(13):2344–8.
Meng S, Yao Z, Liu J, Wang E, Li C, Jiang B, et al. Carbon dots capped cerium oxide nanoparticles for highly efficient removal and sensitive detection of fluoride. J Hazard Mater. 2022;435:128976.
Taniguchi T, Katsumata K-i, Omata S, Okada K, Matsushita N. Tuning growth modes of ceria-based nanocubes by a hydrothermal method. Crystal Growth Design. 2011;11(9):3754–60.
Veitch NC. Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry. 2004;65(3):249–59.
Shi J, Yin T, Shen W. Effect of surface modification on the peroxidase-like behaviors of carbon dots. Colloids Surf, B. 2019;178:163–9.
Henriquez C, Aliaga C, Lissi E. Formation and decay of the ABTS derived radical cation: a comparison of different preparation procedures. Int J Chem Kinet. 2002;34(12):659–65.
Chu X, Shi Q. Versatile magnetic nanoparticles for spatially organized assemblies of enzyme cascades: a comprehensive investigation of catalytic performance. Chin J Chem. 2022;40(12):1437–46.
Nolan M, Ganduglia-Pirovano MV. Enhanced oxidation activity from modified ceria: MnOx–ceria, CrOx–ceria and Mg doped VOx–ceria. Appl Catal B. 2016;197:313–23.
Chen C, Yuan Q, Ni P, Jiang Y, Zhao Z, Lu Y. Fluorescence assay for alkaline phosphatase based on ATP hydrolysis-triggered dissociation of cerium coordination polymer nanoparticles. Analyst. 2018;143(16):3821–8.
Liu H, Li M, Xia Y, Ren X. A turn-on fluorescent sensor for selective and sensitive detection of alkaline phosphatase activity with gold nanoclusters based on inner filter effect. ACS Appl Mater Interfaces. 2017;9(1):120–6.
Goggins S, Naz C, Marsh BJ, Frost CG. Ratiometric electrochemical detection of alkaline phosphatase. Chem Commun. 2015;51(3):561–4.
Jiang H, Wang X. Alkaline Phosphatase-Responsive Anodic Electrochemiluminescence of CdSe Nanoparticles. Anal Chem. 2012;84(16):6986–93.
Liu W, Chu L, Zhang C, Ni P, Jiang Y, Wang B, et al. Hemin-assisted synthesis of peroxidase-like Fe-N-C nanozymes for detection of ascorbic acid-generating bio-enzymes. Chem Eng J. 2021;415:128876.
Li CM, Zhen SJ, Wang J, Li YF, Huang CZ. A gold nanoparticles-based colorimetric assay for alkaline phosphatase detection with tunable dynamic range. Biosens Bioelectron. 2013;43:366–71.
Acknowledgements
This work was financially supported by GDAS Project of Science and Technology Development (2022GDASZH-2022010101), Natural Science Foundation of China (Nos. 21603067, 22073025), and Research and Development Plan in Key Areas of Guangdong Province (No. 2022B1111040002).
Author information
Authors and Affiliations
Contributions
Wang Qian: Conceptualization, Data curation, Writing - Original draft, Writing - Reviewing and editing.
Meng Song: Investigation, Methodology.
Zhou Gang: Funding acquisition, Investigation.
Shi Qingshan: Formal analysis.
Xu Ziqiang: Project administration, Data curation.
Xie Xiaobao: Supervision, Validation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection featuring Nanozymes with guest editors Vipul Bansal, Sudipta Seal, and Hui Wei.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Meng, S., Zhou, G. et al. Polymer-enhanced peroxidase activity of ceria nanozyme for highly sensitive detection of alkaline phosphatase. Anal Bioanal Chem (2024). https://doi.org/10.1007/s00216-024-05307-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00216-024-05307-8