Abstract
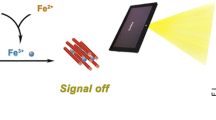
This paper describes initially the application of the Tyndall effect (TE) of metal–organic framework (MOF) materials as a colorimetric signaling strategy for the sensitive detection of pyrophosphate ion (PPi). The used MOF NH2-MIL-101(Fe) was prepared with Fe3+ ions and fluorescent ligands of 2-amino terephthalic acid (NH2-BDC). The fluorescence of NH2-BDC in MOF is quenched due to the ligand-to-metal charge transfer effect, while the NH2-MIL-101(Fe) suspension shows a strong TE. In the presence of PPi analyte, the MOFs will undergo decomposition because of the competitive binding of Fe3+ by PPi over NH2-BDC, resulting in a significant decrease in the TE signal and fluorescence restoration from the released ligands. The results demonstrate that the new method only requires a laser pointer pen (for TE creation) and a smartphone (for portable quantitative readout) to detect PPi in a linear concentration range of 1.25–800 μM, with a detection limit of ~210 nM (3σ) which is ~38 times lower than that obtained from traditional fluorescence with a spectrophotometer (linear concentration range, 50–800 µM; detection limit, 8.15 µM). Moreover, the acceptable recovery of PPi in several real samples (i.e., pond water, black tea, and human serum and urine) ranges from 97.66 to 119.15%.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyrophosphate ion (PPi) is an important biological anion and a byproduct of adenosine triphosphate hydrolysis [1]. It participates in metabolic cycles within the human body and plays a crucial role in many key biological reactions [2,3,4], such as biomacromolecules syntheses [5], bone formation [6], nucleic acid replication [7], telomere maintenance, cell signal transmission, and energy storage [8, 9]. As a biomarker, abnormal levels of PPi are closely associated with certain important diseases including arthritis [10], hypophosphatemia [11], infectious diseases [12], and even cancers [13,14,15]. Additionally, PPi has extensive applications in the food industry and industrial production. It is used for food preservation [16], flavoring, and texture [17, 18] and is often used as a softener and rust remover for water treatment in industry [19,20,21]. Therefore, sensitive and specific detection of PPi holds significant importance for medical diagnosis, food safety control, and environmental monitoring.
Existing methods for the PPi detection mainly include high-performance liquid-chromatography (HPLC) [22], enzymatic assay [23], chemiluminescence technology [24], electrochemical sensor [25], fluorescence technology [26,27,28,29,30,31,32,33], and visual assay [34,35,36,37]. Among them, the instrumental methods (e.g., HPLC, enzymatic assay, chemiluminescence and fluorescence technologies, and electrochemical sensor) could commonly offer satisfactory analytical sensitivity, but they might suffer from some disadvantages such as requirements of expensive equipment, highly skilled operators, or complex analytical procedures to varying degrees, which would highly limit their applications. On the other hand, simple and low-cost visual assays have alternatively attracted increasing attention. In general, such methods use a variety of optical probes to realize naked-eye analysis of PPi, such as luminol-Ga3+ nanoassembles [36], diallyldimethylammonium chloride-Zn2+ nanocomposites [37], fluorescence dyes [38], noble metal nanoparticles (e.g., Au or Ag) [39, 40], carbon dots [41, 42], metal–organic frameworks (MOFs) [43, 44], and so on. In comparison, MOFs are an emerging class of crystalline organic–inorganic hybrid materials with molecular-sized cavities formed by the self-assembly of metal ions/clusters and organic ligands. There are growing uses of MOFs as new effective sensing materials (probes) for chemical and biological analysis in recent years, due to their unique physical and chemical properties like stable crystal structure, abundant binding site, and ease of functionalization. For instance, several groups have successfully prepared some useful luminescent MOFs (e.g., pyridine-functionalized UiO-66-N-Py [45], luminol-loaded MIL-101(Cr) [46], and porphyrin-based PCN-224 decorated with sulfur nanodots [47]) for rapid qualitative “turn-on” or “turn-off” fluorescence determination of PPi based on competitive coordination or photoelectron transfer mechanisms. However, expensive spectrophotometers are still necessary for these methods to realize accurate quantitative measurement of analyte concentrations. In this regard, the development of new MOF-based visual PPi assays, which can achieve the ultimate economical goal of rapid naked-eye qualitative or semi-quantitative judgment and instrument-free quantitative analysis, is a significant but highly challenging task.
In response to this challenge, for the first time, this paper proposes a novel visual method with the above features for PPi detection by utilizing another fundamental non-fluorescent property of MOFs, namely, the Tyndall effect (TE) for colorimetric signaling. TE is a common natural light scattering phenomenon that generally takes place in a solution where it involved nanometer or micrometer-sized inorganic or organic materials or particles (e.g., macromolecules like proteins, colloidal metal particles, MOFs) scatter incident light to form a visible path. The TE’s color depends on the wavelength of the incident light, while its intensity is positively related to the size and concentration of the suspended materials or particles. The recent studies by our group demonstrate that taking colloidal Au and Ag nanoparticles as model probes [48, 49], their TE signals enable better analytical performance for colorimetric assay than their surface plasmon resonance or absorption responses. Herein, this work attempts to initially explore the advantages of MOF’s TE over its fluorescence signal for visual analysis.
Figure 1 shows the schematic working principle of the new PPi assay based on the MOF’s TE. With a solvothermal method, the MOF of NH2-MIL-101(Fe) is synthesized with Fe3+ ions and fluorescent ligands of 2-amine terephthalic acid (NH2-BDC) as raw materials. The fluorescence of NH2-BDC in MOF is quenched due to the ligand-to-metal charge transfer (LMCT) effect after its interaction with Fe3+. The suspension of NH2-MIL-101(Fe) exhibits a strong multi-color (red and yellow or even white) TE signal when irradiated by a 635 nm laser pointer pen as a handheld light source. Upon incubation of a PPi sample, the analyte competes with NH2-BDC for binding to Fe3+, leading to rapid MOF disintegration and a significant decrease in the TE of the reaction solution (accompanied by fluorescence recovery from the NH2-BDC ligand released). The reduction degree of TE is positively proportional to the PPi level in the sample. Further portable quantitative analysis could be performed by using a smartphone to record the TE signal. To achieve the best signal-to-background ratio, the main experimental conditions have been optimized, including the concentration of MOF, reaction time, temperature, and pH. Moreover, a comparative study for evaluating the advantages of MOF’s TE over its fluorescence signal for visual analysis is conducted. The accuracy and practicality of the TE-based PPi assay are also validated by analyzing several real complex samples (i.e., pond water, black tea, human serum, and human urine).
Experimental
Materials
Sodium pyrophosphate (Na4P2O7) and 2-amine terephthalic acid (NH2-BDC) were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Ferric chloride hexahydrate (FeCl3·6H2O) was obtained from Xilong Chemical Co., Ltd. (Guangdong, China). All of the other reagents were of analytical grade. The used buffer is 0.04 M Britton–Robinson buffered saline solution (pH 7). Any other type of buffers could also be used as long as they do not interfere with the interactions between the PPi analyte and MOFs. Hydrophilic polycarbonate nanoporous membrane with an average pore size of approximately 50 nm was a product of GE Healthcare Life Sciences. Real environmental water samples were collected from a pool on campus. Real beverage (Kangshifu black tea) was purchased from a supermarket on campus. Human serum and urine samples from healthy volunteers were collected from the university hospital. Before analysis, real samples were filtered with the nanoporous membrane to remove potential interferents. Unless otherwise specified, all stock solutions and buffers were prepared using ultrapure water with a resistivity ≥18.2 MΩ·cm.
Apparatus
The 635-nm laser pointer pen (5 mW) was purchased from Deli Group Co., Ltd. (Ningbo, China). The ultrapure water system (UPS-II-20L) was obtained from Chengdu Yuejun Technology Co., Ltd. (Chengdu, China). Thermogravimetric analysis of NH2-MIL-101(Fe) was performed using a TA Q500 (TA Instruments, USA). X-ray diffraction (XRD) data were recorded on an X-ray diffraction automated diffractometer from Malvern Panalytical Ltd. (X’Pert3 Power, UK) using CuKα radiation from 5° to 55° at a scan rate of 5°/min. The surface morphologies of NH2-MIL-101(Fe) were investigated using a JSM-5600 LV scanning electron microscope (SEM, JEOL, Japan) and dynamic light scattering instrument from Malvern Instruments Ltd. (Zeta sizer Nano ZS90, UK). Fluorescence spectra were measured with a F-7000 spectrophotometer (Hitachi, Japan). Though a 445-nm (blue) or 520-nm (green) laser pointer pen is also commercially available to create TE, we prefer to use the 635-nm red laser because it shows relatively lower energy and might rarely activate potential fluorescent background interference in real samples. All colorimetric images of TE and fluorescence results were recorded using a Samsung smartphone (Galaxy S5, South Korea).
Preparation of NH2-MIL-101(Fe)
The MOF of NH2-MIL-101(Fe) was prepared by using a reported solvothermal method [50]. In brief, 0.6749 g of FeCl3·6H2O and 0.2249 g of NH2-BDC were dissolved separately in 7.5 mL of N, N-dimethylformamide. The two solutions were mixed thoroughly, followed by ultrasonic treatment for 30 min. Then, the resulting mixture was transferred to a Teflon-lined autoclave for heating treatment at 110 ℃ for 24 h. After its cooling to room temperature, a brown precipitate was collected by centrifugation. The obtained product was washed successively three times with N,N-dimethylformamide and methanol to remove excess unreacted reagents. Subsequently, it was dried at 70 ℃ for 12 h to obtain a reddish brown solid powder of NH2-MIL-101(Fe).
Analytical procedures
For each assay run, 300 μL of artificial sample or real sample was incubated successively with 10 μL of 0.5 M ethylenediaminetetraacetic acid disodium and 300 μL of 0.5 mg/mL NH2-MIL-101(Fe) suspension at room temperature (~25 ℃) for 10 min. The MOFs will undergo decomposition due to the competition binding of Fe3+ ions by the analyte over the NH2-BDC ligands that would be in turn released into the resultant mixture. For the fluorescence method, such reaction solution containing the released NH2-BDC molecules was illuminated by a UV lamp (with excitation wavelength of 365 nm) for simple visual qualitative or semi-quantitative PPi analysis. Moreover, the fluorescence spectrum of the released ligands was recorded during an emission wavelength range of 400–800 nm (with excitation wavelength of 340 nm) for quantitative measurement of PPi concentration.
On the other hand, for the novel TE-based method, a home-made fully-enclosed device, which was capable of providing a stable “black” environmental condition (Fig. S1 in Supplementary Material), was adopted to load the reaction solution. And, the laser pointer pen installed in the device was used to irradiate the solution to generating the TE signal for naked-eye qualitative or semi-quantitative PPi analysis. For quantitative detection, each TE image was captured with smartphone (fixed parameters: ISO 200, WB 6000K, and EV 0) and further processed with the ImageJ software to analyze its average gray (AG) value according to the following three steps: (1) open/reverse the TE image; (2) use “Rectangle” function for selecting its partial area; and (3) select “Analysis” function to obtain the AG value. The ΔAG value for each sample was defined as ΔAG = AGblank − AGPPi, where AGPPi and AGblank represent the values obtained from the PPi sample and a blank sample (buffer without the analyte), respectively. For the selectivity tests, the same procedures above were performed to analyze other types of ions or molecules, i.e., Al3+, Cd2+, Ca2+, Pb2+, Cu2+, Mg2+, Mn2+, Fe2+, Hg2+, Co2+, NH4+, Ag+, SCN−, NO2−, CI−, CH3COO−, HCO3−, Br−, I−, HPO42−, SO42−, PO43−, lysine, L-cysteine, glucose, vitamin C, urea, or glutathione instead of PPi.
Results and discussion
The key concept of the new method focuses on the conversion of PPi detection into the reduction measurement of the MOFs’ TE. Thus, in order to successfully carry out our method, the first challenge is to verify feasibility of PPi-triggered structural disintegration of NH2-MIL-101(Fe). Figure 2A shows that the pure Fe3+ solution (6 mM) exhibits a slight brownish-yellow color (image a1). The NH2-BDC is an excellent organic fluorescent dye; its solution is clear and colorless (Fig. 2A, image a2) but shows bright blue fluorescence under the illumination of a UV lamp at 364 nm (Fig. 2A, image b2). Its characteristic fluorescence emission peak is located at 450 nm (Fig. 2C, curve b2). Neither of the two solutions displays a TE response when irradiated by a 635 nm laser pointer pen (Fig. 2A, images c1–c2).
A Colorimetric (a), fluorescence (b), and TE (c) results recorded from different (reaction) solutions: (1) 6 mM Fe3+, (2) 6 mM NH2-BDC, (3) 0.5 mg/mL NH2-MIL-101(Fe) in buffer, and (4) 0.5 mg/mL NH2-MIL-101 (Fe) incubated with 0.5 mM PPi. B SEM image (left) and particle size (right) distribution results measured from (reaction) solutions a3–a4 shown in A. C Fluorescence spectra measured from solutions b1–b4 are shown in A. D AG values calculated from the TE images c1–c4 shown in A. Each error bar represents a standard deviation across three replicate experiments
After solvothermal reactions between Fe3+ ions and NH2-BDC molecules, a dark brown mixture was obtained (Fig. 2A, image a3). The SEM image and dynamic light scattering characterization of the involved solid products reveal that octahedral-shaped MOFs of NH2-MIL-101(Fe) with an average size of about 850 nm have been formed (Fig. 2B, image a3), which is consistent well with the results reported by Li and co-workers [51]. The XRD measurement demonstrates that these as-prepared particles possess high crystallinity [52] (Fig. S2 in Supplementary Material). The thermogravimetric analysis further shows that their material loss rate was less than 50% at a high temperature of 500 ℃, suggesting good thermal stability of such MOFs (Fig. S3 in Supplementary Material). In addition, both of optical image (under UV lamp illumination) and fluorescence spectrum indicate that the fluorescence of NH2-BDC in the MOFs in the dark brown mixture has been completely quenched (Fig. 2A, image b3; Fig. 2C, curve b3). This may be attributed to the LMCT process that occurred in the Fe3-µ3-oxo clusters in the MOF network structure [53, 54]. Interestingly, as there are lots of sub-micrometer-sized particles in the MOF suspension that could efficiently scatter the 635-nm laser beam, a strong red TE signal even with a white–yellow mixed light path in the middle is observed in the brown solution (Fig. 2A, image c3). Its AG value exceeds ~140 (Fig. 2D, image c3).
However, after the suspension was incubated with a PPi sample (500 μM), its original color changed from dark brown to slight brownish (Fig. 2A, image a4), and the fluorescence of NH2-BDC was significantly restored (Fig. 2A, image b4; Fig. 2C, curve b4). Moreover, its TE response decreased sharply; only a red light path was found (Fig. 2A, image c4), with a corresponding AG value reduced dramatically to around ~40 (Fig. 2D, image c4). This observation implies that PPi might show a higher affinity towards Fe3+ compared to NH2-BDC through forming Fe-O-P bonds. Such competition reactions immediately mediated in situ disintegration of MOFs leading to a drastic reduction in the number of particles effectively scattering the laser beam (Fig. 2B, image a4). Their average size decreased to approximately 350 nm (Fig. 2B, image a4). At the same time, a large number of fluorescent ligands of NH2-BDC were released into the solution (Fig. 2B, image b4).
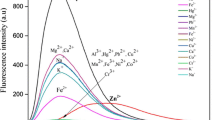
After demonstrating the PPi-triggered disintegration and TE reduction of the MOFs, the main experimental conditions have been optimized to achieve the best analytical performance, such as the NH2-MIL-101(Fe) concentration, reaction pH, temperature, and time (Figs. S4-S7 in Supplementary Material). Subsequently, under the optimized experimental conditions, the new method was used to comparatively detect blank sample (buffer without PPi), 500 μM PPi, and 28 types of other possible interfering anions (SCN−, NO2−, CI−, CH3COO−, HCO3−, Br−, I−, HPO42−, SO42−, or PO43−; 5 mM each), cations (Al3+, Cd2+, Ca2+, Pb2+, Cu2+, Mg2+, Mn2+, Fe2+, Hg2+, Co2+, NH4+, or Ag+; 5 mM each), and small molecules (lysine, L-cysteine, glucose, vitamin C, urea, or glutathione; 5 mM each) to study its analytical selectivity (Fig. 3). One can clearly find from Fig. 3 that only the assay of PPi sample resulted in a quite weak TE signal with a low AG value of about 70, while the intense TE responses and high AG values (from ~132 to ~150) measured in the analysis of other samples were very similar to that obtained in the case of blank sample (with an AG value up to ca. 143). Moreover, the 28 types of tested possible interfering substances (5 mM) also showed no effects on the TE response and AG value obtained for the PPi detection (Fig. S8 in Supplementary Material). The results suggest that the developed method has good detection selectivity, namely, the PPi specifically mediated the decrease in the TE signal. The other 28 sorts of anions, cations, and small molecules could not effectively interact with Fe3+ or NH2-BDC, and as a result, the MOFs in each case could maintain their original structures efficiently scattering laser beams to generate strong TE.
Next, to evaluate the analytical advantages of the new TE-based method over the traditional fluorescence technique, both methods were simultaneously used to detect a series of PPi samples with different analyte concentrations. The comparison results are shown in Fig. 4. From Fig. 4A, it can be observed that within the tested level range from 0 to 100 µM, as the analyte concentration increases, the TE intensity gradually decreases (Fig. 4A, top), showing clear yet reverse PPi-regulated visual responses. On the other hand, the fluorescence intensity of the reaction mixtures increases with the increasing analyte concentration (Fig. 4A, bottom). These results indirectly reflect that higher concentrations of PPi could make more NH2-MIL-101(Fe) disintegrate to release more fluorescent NH2-BDC ligands. Meanwhile, fewer MOFs were remaining in the reaction solutions for scattering the red laser beam, thus resulting in a lower TE intensity. Furthermore, the concentrations of ~5 and ~100 µM were defined as the visual limits of detection (LODs) for the TE-based new method and traditional fluorescence one, respectively, because both TE and fluorescence signals recorded from the two corresponding reaction mixtures became significantly distinguishable from that produced in the analysis of blank samples (Fig. 4A, top and bottom, images 0–4)
A Visual TE (top) and fluorescence (bottom) results obtained from the assays of PPi samples with different analyte concentrations: (1) 0, (2) 1.25, (3) 2.5, (4) 5, (5) 20, (6) 50, (7) 100, (8) 200, (9) 300, (10) 400, (11) 500, (12) 600, (13) 700, (14) 800, (15) 900, and (16) 1000 μM of PPi. B Fluorescence spectra recorded from the reaction mixtures shown in A (bottom). C Calibration curves describing the relationships between the PPi concentration and the AG change (AGblank − AGPPi) of the TE results shown in A, (top) or the fluorescence intensity at 445 nm measured in the spectra shown in B. Each error bar represents one standard deviation for three replicate experiments
More interestingly, Fig. 4A further reveals that the TE response could offer a better semi-quantitative analytical advantage over the traditional fluorescence signal for colorimetric signaling particularly in terms of higher concentration resolution. For example, as for the fluorescence signaling, the semi-quantitative analysis primarily relied on the relative intensity of the single, uniform fluorescence signal from the entire homogeneous reaction solution. As a result, it was unable to visually distinguish the fluorescence responses recorded from the three PPi samples at medium levels from 100 to 300 µM (Fig. 4A, bottom images 7–9). Additionally, almost the same fluorescence signals were also produced when detecting other seven PPi samples containing higher concentrations in the range of 400–1000 µM (Fig. 4A, bottom images 10–16). In contrast, the new method allowed for clear visual differentiation of TE responses from thirteen PPi samples with a quite wide concentration range of 1.25–800 µM (Fig. 4A, top images 3–14). This could be attributed to the basic fact in that the TE exhibited distinct adaptive multi-color responses. That is, for the assays of PPi samples with low concentrations (from 1.25 to 200 µM), red–yellow–white three-color or red–yellow dual-color TE responses were produced; the width of the white or yellow light path in the middle was negatively proportional to the analyte level (Fig. 4A, top images 1–8). And, in the cases of PPi samples of higher levels (300–800 μM), single-color red TE signals were obtained, whose red light path width reversely depended on the analyte concentration (Fig. 4A, top images 9–16).
The quantitative analytical performance of both methods was also investigated comparatively. Figure 4C displays the fluorescence spectra recorded from the assays of PPi samples with tested levels from 0 to 100 µM. It can be observed that with an increase in PPi concentration, larger amounts of MOF were degraded to release more NH2-BDC molecules showing higher intensity of fluorescence responses. The fluorescence intensity of NH2-BDC measured at 445 nm exhibits a linear relationship with analyte concentrations within the range of 50–800 µM (Fig. 4D, blue line), with a regression equation of y = 0.87907x – 29.60579 (R2 = 0.99165). According to the 3σ rule, the quantitative LOD for PPi was estimated to be approximately 8.15 μM. Moreover, the intra-/inter-day precisions and accuracies of the fluorescence method were investigated. Relative standard deviations (RSDs) obtained from the intra-day and inter-day assays of 100, 200 and 500 µM PPi samples were 5.47, 6.93, and 9.51% (n = 5) and 3.17, 7.83, and 8.86% (n = 7), respectively.
For the new method, the TE image of each reaction mixture was captured by a smartphone to calculate its ΔAG value (AGblank − AGPPi). Figure 4D shows that the ΔAG value was linearly related to the PPi levels from 1.25 to 800 μM (Fig. 4D, red line), with a regression equation of y = 0.14315x + 11.83472 (R2 = 0.99509). A quantitative LOD as low as ~210 nM was achieved (3σ), which is about 38 times lower than that obtained by the traditional fluorescence method (8.15 μM). In addition, RSDs obtained from the intra-day and inter-day assays of 100, 200, and 500 µM PPi samples were 3.44, 1.71, and 2.00% (n = 5) and 2.70, 3.20, and 2.40% (n = 7), respectively, indicating satisfactory detection precisions and accuracies of the proposed method. In comparison with some other recently reported methods for PPi detection using other fluorescent or colorimetric probes (e.g., boron-doped carbon nanodots, N-doped carbon dots, DNA-attached cobalt oxyhydroxide, dual-emissive carbon dot) with large expensive spectrophotometer readers, the proposed equipment-free TE-based method could also achieve comparable or even better detection performance (Table 1).
Finally, to evaluate the practicality of the new method, it was applied to detect PPi in several real samples, i.e., pond water, black tea, human serum, and human urine. As shown in Table 2, it can be found that the recovery of different concentrations of PPi obtained in these real samples is between 97.66 and 119.15%, with RSD from multiple parallel analysis ranges from 0.59 to 3.26% (n = 3). These recovery results and analytical deviations confirm acceptable detection accuracy and practicality of the TE-based equipment-free quantitative PPi assay developed herein.
Conclusion
We have successfully demonstrated the use of multi-color TE response of MOF (NH2-MIL-101(Fe)) rather than fluorescence signal for the amplified colorimetric signaling to realize visual qualitative/semi-quantitative analysis and equipment-free quantification of PPi. The analyte can induce rapid degradation of NH2-MIL-101(Fe), leading to a significant decrease in its naked-eye TE response. The results reveal that the new PPi assay only requires a user-friendly, cheap laser pointer pen and a ubiquitous smartphone to quickly finish an assay run within 10 min. Furthermore, its detection sensitivity is approximately 38 times higher than that of the traditional fluorescence method with an expensive spectrophotometer. More importantly, as for visual analysis, in comparison with the fluorescence method relying on the intensity of a single uniform fluorescence signal from the entire homogeneous reaction solution, the TE signaling provides a far superior semi-quantitative level resolution due to its inherent unique multi-color responding mode. Therefore, with further iterative optimization in reaction platform and data processing app, the proposed non-instrumental quantitative method has great potential for wide applications like portable, field-based PPi analysis in various fields including environmental monitoring, food safety, and medical diagnosis. It is also worth noting that the TE signaling strategy possesses good universality theoretically; it is expected to be applied to analytical systems of other types of MOFs in which an analyte can mediate their in situ disintegration (or synthesis), on which some research work is now underway in our group. Some of future works will focus on the (1) regulation of the MOFs’ concentrations for zooming the dynamic determination ranges and (2) development of customized apps (with functions like TE imaging and data processing) in addition to introduction of reference signals to minimize the effect of mobile models.
References
Stetten MR. Metabolism of inorganic pyrophosphate. J Biol Chem. 1964;239:3576–83. https://doi.org/10.1016/s0021-9258(18)97761-6.
Lee S, Yuen KK, Jolliffe KA, Yoon J. Fluorescent and colorimetric chemosensors for pyrophosphate. Chem Soc Rev. 2015;44:1749–62. https://doi.org/10.1039/c4cs00353e.
Chen CY, Tan YZ, Hsieh PH, Wang CM, Shibata H, Maejima K, et al. Metal-free colorimetric detection of pyrophosphate ions by inhibitive nanozymatic carbon dots. ACS Sens. 2020;5:1314–24. https://doi.org/10.1021/acssensors.9b02486.
Dong C, Ma X, Qiu N, Zhang Y, Wu A. An ultra-sensitive colorimetric sensor based on smartphone for pyrophosphate determination. Sens Actuators B: Chem. 2021;329:129066. https://doi.org/10.1016/j.snb.2020.129066.
Anbu S, Paul A, Stasiuk GJ, Pombeiro AJL. Recent developments in molecular sensor designs for inorganic pyrophosphate detection and biological imaging. Coordin Chem. 2021;431:213744. https://doi.org/10.1016/j.ccr.2020.213744.
Kim S, Han J, Chung H, Choi YK, Hashkavayi AB, Zhou Y, et al. Pyrophosphate-enhanced oxidase activity of cerium oxide nanoparticles for colorimetric detection of nucleic acids. Sensors. 2021;21:7567. https://doi.org/10.3390/s21227567.
Xu SQ, He M, Yu HP, Wang XY, Tan XL, Lu B, et al. Bioluminescent method for detecting telomerase activity. Clin Chem. 2002;48:1016–20. https://doi.org/10.1093/clinchem/48.7.1016.
Cowart RE, Swope S, Loh TT, Chasteen ND, Bates GW. The exchange of Fe3+ between pyrophosphate and transferrin. Probing the nature of an intermediate complex with stopped flow kinetics, rapid multimixing, and electron paramagnetic resonance spectroscopy. J Biol Chem. 1986;261:4607–14. https://doi.org/10.1016/s0021-9258(17)38545-9.
Greenfield TJ, Julve M, Doyle RP. Exploring the biological, catalytic, and magnetic properties of transition metal coordination complexes incorporating pyrophosphate. Coordin Chem. 2019;384:37–64. https://doi.org/10.1016/j.ccr.2018.12.011.
Tsui FWL. Genetics and mechanisms of crystal deposition in calcium pyrophosphate deposition disease. Curr Rheumatol Rep. 2011;14:155–60. https://doi.org/10.1007/s11926-011-0230-6.
Nonaka A, Horie S, James TD, Kubo Y. Pyrophosphate-induced reorganization of a reporter–receptor assembly via boronate esterification; a new strategy for the turn-on fluorescent detection of multi-phosphates in aqueous solution. Org Biomol Chem. 2008;6:3621–5. https://doi.org/10.1039/b808027e.
Yang Y, Chen P, Liu Y, Cai Z, Wang X, Me Y, et al. A colorimetric indicator-displacement assay based on stable Cu2+ selective carbon dots for fluorescence turn-on detection of pyrophosphate anions in urine. Spectrochim Acta A. 2021;251:119479. https://doi.org/10.1016/j.saa.2021.119479.
Harrington CT, Lin EI, Olson MT, Eshleman JR. Fundamentals of pyrosequencing. Arch Pathol Lab. 2013;137:1296–303. https://doi.org/10.5858/arpa.2012-0463-RA.
Wongkongkatep J, Ojida A, Hamachi I. Fluorescence sensing of inorganic phosphate and pyrophosphate using small molecular sensors and their applications. Topics Curr Chem. 2017;375:30. https://doi.org/10.1007/s41061-017-0120-0.
An X, Tan Q, Pan S, Liu H, Hu X. A turn-on luminescence probe based on amino-functionalized metal-organic frameworks for the selective detections of Cu2+, Pb2+ and pyrophosphate. Spectrochim Acta A. 2021;247:119073. https://doi.org/10.1016/j.saa.2020.119073.
Lanne ABM, Goode A, Prattley C, Kumari D, Drasbek MR, Williams P, et al. Molecular recognition of lipopolysaccharide by the lantibiotic nisin. BBA-Biomembranes. 2019;1861:83–92. https://doi.org/10.1016/j.bbamem.2018.10.006.
Yu Q, Peng Y, Cao Q, Ma J, Shi Z, Jia Q. Pyridinaldehyde modified luminescence metal-organic framework for highly sensitive and selective fluorescence detection of pyrophosphate. Sens Actuators B: Chem. 2022;365:131949. https://doi.org/10.1016/j.snb.2022.131949.
Jin H, Chen J, Zhang J, Sheng L. Impact of phosphates on heat-induced egg white gel properties: texture, water state, micro-rheology and microstructure. Food Hydrocolloids. 2021;110:106200. https://doi.org/10.1016/j.foodhyd.2020.106200.
Lin Z, Luo S, Xu D, Liu S, Wu N, Yao W, et al. Silica-polydopamine hybrids as light-induced oxidase mimics for colorimetric detection of pyrophosphate. The Analyst. 2020;145:424–33. https://doi.org/10.1039/c9an01813a.
Xu H, Zhu X, Dong Y, Wu H, Chen Y, Chi Y. Highly sensitive electrochemiluminescent sensing platform based on graphite carbon nitride nanosheets for detection of pyrophosphate ion in the synovial fluid. Sens Actuators B: Chem. 2016;236:8–15. https://doi.org/10.1016/j.snb.2016.05.056.
Sronsri C, Sittipol W, U-yen K. Optimization of biodiesel production using magnesium pyrophosphate. Chem Eng Sci. 2020;226:115884. https://doi.org/10.1016/j.ces.2020.115884.
Ito M, Fujii N, Wittwer C, Sasaki A, Tanaka M, Bittner T, et al. Hydrophilic interaction liquid chromatography–tandem mass spectrometry for the quantitative analysis of mammalian-derived inositol poly/pyrophosphates. J Chromatogr A. 2018;1573:87–97. https://doi.org/10.1016/j.chroma.2018.08.061.
Nguyen VL, Darman M, Ireland A, Fitzpatrick M. A high performance liquid chromatography fluorescence method for the analysis of both pyridoxal-5-phosphate and thiamine pyrophosphate in whole blood. Clin Chim Acta. 2020;506:129–34. https://doi.org/10.1016/j.cca.2020.03.026.
Li F, Liu Y, Li Z, Li Q, Liu X, Cui H. Cu(II)-regulated on-site assembly of highly chemiluminescent multifunctionalized carbon nanotubes for inorganic pyrophosphatase activity determination. ACS Appl Mater Interfaces. 2020;12:2903–9. https://doi.org/10.1021/acsami.9b20259.
Xu W, Shao C, Pang J, Jiang Y, Han Y, Wang J. Electrochemical method of pyrophosphate determination by quinone reduction. Electrochim Acta. 2019;300:171–6. https://doi.org/10.1016/j.electacta.2019.01.096.
Xu HR, Li K, Jiao SY, Li LL, Pan SL, Yu XQ. Tetraphenylethene based zinc complexes as fluorescent chemosensors for pyrophosphate sensing. Chinese Chem Lett. 2015;26:877–80. https://doi.org/10.1016/j.cclet.2015.05.037.
Wang ZX, Yu XH, Li F, Kong FY, Lv W-X, Fan DH, et al. Preparation of boron-doped carbon dots for fluorometric determination of Pb(II), Cu(II) and pyrophosphate ions. Microchim Acta. 2017;184:4775–83. https://doi.org/10.1007/s00604-017-2526-3.
Li N, Zhu YD, Liu T, Liu SG, Lin SM, Shi Y, et al. Turn-on fluorescence detection of pyrophosphate anion based on DNA-attached cobalt oxyhydroxide. New J Chem. 2017;41:1993–6. https://doi.org/10.1039/c6nj03491h.
Zhu W, Huang X, Guo Z, Wu X, Yu H, Tian H. A novel NIR fluorescent turn-on sensor for the detection of pyrophosphate anion in complete water system. Chem Commun. 2012;48:1784–6. https://doi.org/10.1039/c2cc16902a.
Selva Sharma A, Suresh Nair S, Varghese AW, Usha A, Varghese RE, Joseph R, et al. Dual-emissive carbon dots: exploring their fluorescence properties for sensitive turn-off-on recognition of ferric and pyrophosphate ions and its application in fluorometric detection of the loop-mediated isothermal amplification reaction. Langmuir. 2023;39:5779–92. https://doi.org/10.1021/acs.langmuir.3c00041.
Zhang WJ, Liu SG, Han L, Luo HQ, Li NB. A ratiometric fluorescent and colorimetric dual-signal sensing platform based on N-doped carbon dots for selective and sensitive detection of copper(II) and pyrophosphate ion. Sens Actuators B: Chem. 2019;283:215–21. https://doi.org/10.1016/j.snb.2018.12.012.
Das A, De S, Das G. Naphthyl-functionalized ninhydrin-derived receptor for ‘CHEF’-based sequential sensing of Al(III) and PPi: prospective chemosensing applications under physiological conditions. J Photoch Photobio A. 2021;418:113422. https://doi.org/10.1016/j.jphotochem.2021.113442.
Dai Y, Liu Z, Bai Y, Chen Z, Qin J, Feng F. A novel highly fluorescent S, N, O co-doped carbon dots for biosensing and bioimaging of copper ions in live cells. RSC Adv. 2018;8:42246–52. https://doi.org/10.1039/c8ra09298b.
Kong C, Liu Q, Li W, Chen Z. Single particle-based colorimetric assay of pyrophosphate ions and pyrophosphatase with dark-field microscope. Sens Actuators B: Chem. 2019;299:126999. https://doi.org/10.1016/j.snb.2019.126999.
Shi D, Sun Y, Lin L, Shi C, Wang G, Zhang X. Naked-eye sensitive detection of alkaline phosphatase (ALP) and pyrophosphate (PPi) based on a horseradish peroxidase catalytic colorimetric system with Cu(II). Analyst. 2016;141:5549–54. https://doi.org/10.1039/c6an01124a.
Tian X, Qi W, Zhao M, Lai J, Wu D, Hu L, et al. One-pot synthesis of luminol–gallium nanoassemblies and their peroxidase-mimetic activity for colorimetric detection of pyrophosphate. New J Chem. 2020;44:21176–82. https://doi.org/10.1039/d0nj02628j.
Inoue K, Aikawa S, Fukushima Y. Colorimetric detection of pyrophosphate in aqueous solution by pyrogallol red-based Zn2+ complex in the presence of poly(diallyldimethylammonium chloride). Polym Bull. 2018;76:1641–9. https://doi.org/10.1007/s00289-018-2461-4.
Zhang S, Qiao M, Liu T, Ding L, Fang Y. Construction of naphthalimide-based fluorescent amphiphilic aggregates and sensitive detection of persulfate and pyrophosphate anions. Sens Actuators B: Chem. 2022;365:131931. https://doi.org/10.1016/j.snb.2022.131931.
Luo L, Chen Y, Zhang L, Li Y, Li H, Zhang H, et al. SERS assay for pyrophosphate based on its competitive binding to Cu(II) ion on silver nanoparticles modified with cysteine and rhodamine 6G. Microchim Acta. 2016;184:595–601. https://doi.org/10.1007/s00604-016-2044-8.
Cao HT, Zhao T, Liu MQ, Guo LZ, He YQ, Zhang K, et al. Ratiometric optical detection of pyrophosphate based on aggregation-caused dual-signal response of gold nanoclusters. Luminescence. 2023;38:1458–64. https://doi.org/10.1002/bio.4527.
Jin L, Zhao C, Wang X, Zhang Q, Jiang Y, Shen J. Metal-free auxiliary pyrophosphate detection based on near-infrared carbon dots. Spectrochim Acta A. 2023;295:122580. https://doi.org/10.1016/j.saa.2023.122580.
Gao Y, Jiao Y, Zhang H, Lu W, Liu Y, Han H, et al. One-step synthesis of a dual-emitting carbon dot-based ratiometric fluorescent probe for the visual assay of Pb2+ and PPi and development of a paper sensor. J Mater Chem B. 2019;7:5502–9. https://doi.org/10.1039/c9tb01203f.
Hou L, Huang Y, Lin T, Ye F, Zhao S. A FRET ratiometric fluorescence biosensor for the selective determination of pyrophosphate ion and pyrophosphatase activity based on difunctional Cu-MOF nanozyme. Biosens Bioelectron. 2022;10:100101. https://doi.org/10.1016/j.biosx.2021.100101.
Li H, Ren J, Xu X, Ning L, Tong R, Song Y, et al. A dual-responsive luminescent metal-organic framework as a recyclable luminescent probe for the highly effective detection of pyrophosphate and nitrofurantoin. Analyst. 2019;144:4513–9. https://doi.org/10.1039/c9an00718k.
Helal A, Arafat ME, Rahman MM. Pyridinyl conjugate of UiO-66-NH2 as chemosensor for the sequential detection of iron and pyrophosphate ion in aqueous media. Chemosensors. 2020;8:122. https://doi.org/10.3390/chemosensors8040122.
Yu H, Long D. Highly chemiluminescent metal-organic framework of type MIL-101(Cr) for detection of hydrogen peroxide and pyrophosphate ions. Microchim Acta. 2016;183:3151–7. https://doi.org/10.1007/s00604-016-1963-8.
Ma J, Wang S, Wang T, Ma J, Wang Z. Ratiometric fluorescence assay for pyrophosphate based on sulfur nanodots decorated metal-organic frameworks. Chemistry. 2023;29:e202300366. https://doi.org/10.1002/chem.202300366.
Xiao W, Deng Z, Huang J, Huang Z, Zhuang M, Yuan Y, et al. Highly sensitive colorimetric detection of a variety of analytes via the Tyndall effect. Anal Chem. 2019;91:15114–22. https://doi.org/10.1021/acs.analchem.9b03824.
Huang J, Mo X, Fu H, Sun Y, Gao Q, Chen X, et al. Tyndall-effect-enhanced supersensitive naked-eye determination of mercury (II) ions with silver nanoparticles. Sens Actuators B: Chem. 2021;344:130218. https://doi.org/10.1016/j.snb.2021.130218.
Zorainy MY, Gar Alalm M, Kaliaguine S, Boffito DC. Revisiting the MIL-101 metal-organic framework: design, synthesis, modifications, advances, and recent applications. J Mater Chem A. 2021;9:22159–217. https://doi.org/10.1039/d1ta06238g.
Li S, Hu X, Chen Q, Zhang X, Chai H, Huang Y. Introducing bifunctional metal-organic frameworks to the construction of a novel ratiometric fluorescence sensor for screening acid phosphatase activity. Biosens Bioelectron. 2019;137:133–9. https://doi.org/10.1016/j.bios.2019.05.010.
Hu H, Zhang H, Chen Y, Ou H. Enhanced photocatalysis using metal-organic framework MIL-101(Fe) for organophosphate degradation in water. Environ Sci Pollut R. 2019;26:24720–32. https://doi.org/10.1007/s11356-019-05649-2.
Wang X, Yun Y, Sun W, Lu Z, Tao X. A high-performance fluorescence immunoassay based on pyrophosphate-induced MOFs NH2-MIL-88B(Fe) hydrolysis for chloramphenicol detection. Sens Actuators B: Chem. 2022;353:1131143. https://doi.org/10.1016/j.snb.2021.131143.
Xie D, Ma Y, Gu Y, Zhou H, Zhang H, Wang G, et al. Bifunctional NH2-MIL-88(Fe) metal-organic framework nanooctahedra for highly sensitive detection and efficient removal of arsenate in aqueous media. J Mater Chem A. 2017;5:23794–804. https://doi.org/10.1039/c7ta07934f.
Acknowledgements
This work was financially supported by the Central Government-Guided Local Science and Technology Development Project (No. GuikeZY20198006), the Guangxi Scholarship Fund of Guangxi Education Department (No. Guijiaoren-2019-5), the National Natural Science Foundation of China (Nos. 21874032 and 21765007), and the Guangxi Science Fund for Distinguished Young Scholars (No. 2018GXNSFFA281002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All the human serum and urine samples in this study were collected from the Affiliated Hospital of Guilin University of Technology with a protocol approved by the Review Board and Ethical Committee of Guilin University of Technology.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Yan, Y., Zhang, L. et al. Simple, sensitive, colorimetric detection of pyrophosphate via the analyte-triggered decomposition of metal–organic frameworks regulating their adaptive multi-color Tyndall effect. Anal Bioanal Chem 416, 1821–1832 (2024). https://doi.org/10.1007/s00216-024-05200-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-024-05200-4