Abstract
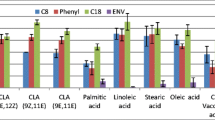
Lipids in human colostrum provide the majority of energy intake and essential fatty acids for developing infants. The fatty acid composition of human colostrum is highly variable and influenced by multiple factors. Human colostrum is a complex sample bringing challenges to fatty acid profiling. This work aimed to optimize the use of ionic liquid (IL) columns and flow-modulated comprehensive two-dimensional gas chromatography coupled to mass spectrometry (FM-GC×GC-MS) for fatty acid profiling in human colostrum. Derivatization strategies were optimized and the elution behavior of fatty acid methyl esters (FAME) on various 1D column phases (Solgel-WAX, SLB-IL60i, SLB-IL76i, and SLB-IL111i). Derivatization with sodium methoxide yielded a satisfactory recovery rate (90%) at milder conditions and reduced time. The use of IL60 as the 1D column provided superior separation, good peak shape, and better utilization of elution space. As a proof of concept, the developed method was applied to access the effects of the mode of neonatal delivery (vaginal vs. C-section) on the fatty acid profile of human colostrum samples. The integrated multidimensional gas chromatography strategy improved FAME detection and separation and can be a useful tool for accessing the effects of different factors on the fatty acid profiling of complex samples.







Similar content being viewed by others
References
Floris LM, Stahl B, Abrahamse-Berkeveld M, Teller IC. Human milk fatty acid profile across lactational stages after term and preterm delivery: a pooled data analysis. Prostaglandins, Leukotrienes Essen Fatty Acids. 2020;156:102023. https://doi.org/10.1016/j.plefa.2019.102023.
Hanley B, Dijane J, Fewtrell M, Grynberg A, Hummel S, Junien C, Koletzko B, Lewis S, Renz H, Symonds M, Gros M, Harthoorn L, Mace K, Samuels F, van der Beek EM. Metabolic imprinting, programming and epigenetics – a review of present priorities and future opportunities. British J Nutri. 2010;104:S1–S25. https://doi.org/10.1017/S0007114510003338.
(1982) ESPGAN committee on nutrition. Guidelines on infant nutrition. III. Recommendations for infant feeding. Acta Paediatr Scand Suppl 302:1–27.
Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE. Essential fatty acids in visual and brain development. Lipids. 2001;36:885–95. https://doi.org/10.1007/s11745-001-0798-1.
Gibson RA, Kneebone GM. Fatty acid composition of human colostrum and mature breast milk. Am J Clin Nutr. 1981;34:252–7. https://doi.org/10.1093/ajcn/34.2.252.
Jensen RG. Lipids in human milk. Lipids. 1999;34:1243–71. https://doi.org/10.1007/s11745-999-0477-2.
Koletzko B, Rodriguez-Palmero M, Demmelmair H, Fidler N, Jensen R, Sauerwald T. Physiological aspects of human milk lipids. Early Hum Dev. 2001;65(Suppl):S3–S18. https://doi.org/10.1016/s0378-3782(01)00204-3.
Samuel TM, Thielecke F, Lavalle L, Chen C, Fogel P, Giuffrida F, Dubascoux S, Martínez-Costa C, Haaland K, Marchini G, Agosti M, Rakza T, Costeira MJ, Picaud J-C, Billeaud C, Thakkar SK. Mode of neonatal delivery influences the nutrient composition of human milk: results from a multicenter European Cohort of Lactating Women. Front Nutri. 2022;9 https://doi.org/10.3389/fnut.2022.834394.
Kohn G, van der Ploeg P, Möbius M, Sawatzki G. Influence of the derivatization procedure on the results of the gaschromatographic fatty acid analysis of human milk and infant formulae. Z Ernahrungswiss. 1996;35:226–34. https://doi.org/10.1007/BF01625685.
Zeng AX, Chin S-T, Marriott PJ. Integrated multidimensional and comprehensive 2D GC analysis of fatty acid methyl esters. J Separation Sci. 2013;36:878–85. https://doi.org/10.1002/jssc.201200923.
Bogusz S Jr, Hantao LW, Braga SC, de Matos França VD, da Costa MF, Hamer RD, Ventura DF, Augusto F. Solid-phase microextraction combined with comprehensive two-dimensional gas chromatography for fatty acid profiling of cell wall phospholipids. J Separation Sci. 2012;35:2438–44. https://doi.org/10.1002/jssc.201200256.
Delmonte P, Fardin-Kia AR, Kramer JKG, Mossoba MM, Sidisky L, Tyburczy C, Rader JI. Evaluation of highly polar ionic liquid gas chromatographic column for the determination of the fatty acids in milk fat. J Chromatog A. 2012;1233:137–46. https://doi.org/10.1016/j.chroma.2012.02.012.
Christie WW. Gas chromatography and lipids: a practical guide. Repr: The Oily Press, Ayr, Scotland; 1994.
Delmonte P, Fardin Kia A-R, Kramer JKG, Mossoba MM, Sidisky L, Rader JI. Separation characteristics of fatty acid methyl esters using SLB-IL111, a new ionic liquid coated capillary gas chromatographic column. J Chromatog A. 2011;1218:545–54. https://doi.org/10.1016/j.chroma.2010.11.072.
Ragonese C, Tranchida PQ, Dugo P, Dugo G, Sidisky LM, Robillard MV, Mondello L. Evaluation of use of a dicationic liquid stationary phase in the fast and conventional gas chromatographic analysis of health-hazardous C18 cis/trans fatty acids. Anal Chem. 2009;81:5561–8. https://doi.org/10.1021/ac9007094.
Manzano P, Arnáiz E, Diego JC, Toribio L, García-Viguera C, Bernal JL, Bernal J. Comprehensive two-dimensional gas chromatography with capillary flow modulation to separate FAME isomers. J Chromatog A. 2011;1218:4952–9. https://doi.org/10.1016/j.chroma.2011.02.002.
von Mühlen C, Zini CA, Caramão EB, Marriott PJ. Caracterização de amostras petroquímicas e derivados utilizando cromatografia gasosa bidimensional abrangente (GCxGC). Quím Nova. 2006;29:765–75. https://doi.org/10.1590/S0100-40422006000400025.
Purcaro G, Tranchida PQ, Mondello L. Comprehensive gas chromatography methodologies for the analysis of lipids. In: Handbook of advanced chromatography/mass spectrometry techniques, Chapter 11. AOCS Press: Published by Elsevier Inc.; 2017. https://doi.org/10.1016/B978-0-12-811732-3.00011-X.
Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biolog Chem. 1957;226:497–509. https://doi.org/10.1016/S0021-9258(18)64849-5.
Guidoni M, de Christo Scherer MM, Figueira MM, Schmitt EFP, de Almeida LC, Scherer R, Bogusz S, Fronza M. Fatty acid composition of vegetable oil blend and in vitro effects of pharmacotherapeutical skin care applications. Braz J Med Biol Res. 2019;52:e8209. https://doi.org/10.1590/1414-431X20188209.
Shantha NC, Napolitano GE. Gas chromatography of fatty acids. Journal of Chromatography A. 1992;624:37–51. https://doi.org/10.1016/0021-9673(92)85673-H.
Giuffrida F, Fleith M, Goyer A, Samuel TM, Elmelegy-Masserey I, Fontannaz P, Cruz-Hernandez C, Thakkar SK, Monnard C, De Castro CA, Lavalle L, Rakza T, Agosti M, Al-Jashi I, Pereira AB, Costeira MJ, Marchini G, Vanpee M, Stiris T, et al. Human milk fatty acid composition and its association with maternal blood and adipose tissue fatty acid content in a cohort of women from Europe. Eur J Nutr. 2022;61:2167–82. https://doi.org/10.1007/s00394-021-02788-6.
Delplanque B, Gibson R, Koletzko B, Lapillonne A, Strandvik B. Lipid quality in infant nutrition: current knowledge and future opportunities. J Pediatr Gastroenterol Nutr. 2015;61:8–17. https://doi.org/10.1097/MPG.0000000000000818.
Western RJ, Lau SSG, Marriott PJ, Nichols PD. Positional and geometric isomer separation of FAME by comprehensive 2-D GC. Lipids. 2002;37:715–24. https://doi.org/10.1007/s11745-002-0953-8.
Innis SM. Human milk: maternal dietary lipids and infant development. Proc Nutri Soc. 2007;66:397–404. https://doi.org/10.1017/S0029665107005666.
Fidler N, Koletzko B. The fatty acid composition of human colostrum. Eur J Nutr. 2000;39:31–7. https://doi.org/10.1007/s003940050073.
Acknowledgements
The authors thank the Coordination for the Improvement of Higher Education Personnel (CAPES) foundation for the PNPD/CAPES postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
FFGD: methodology, formal analysis, writing—original draft preparation, review and editing; SBJ: conceptualization, supervision, writing—review and editing; RSS: resources and samples, review and editing; MF; resources and samples, review and editing; LWH: conceptualization writing—review and editing, supervision, project administration funding acquisition.
Corresponding authors
Ethics declarations
Institutional review board
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) (no. 1,804,463).
Informed consent
Patient consent was waived due to the nature of the study. No identification can be made by the results shown herein.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 22 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dias, F.F.G., Bogusz, S., Silva, R.S. et al. Leveraging the use of ionic liquid capillary columns and GC×GC-MS for fatty acid profiling in human colostrum samples. Anal Bioanal Chem 416, 191–201 (2024). https://doi.org/10.1007/s00216-023-05006-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-05006-w