Abstract
Novel optical sensors for nickel determination by incorporation of 5-(2`-bromo-phenylazo)-6-hydroxypyrimidine-2,4-dione (I), 5-(2`,4`-dimethylphenylazo)-6-hydroxypyrimidine-2,4-dione (II), dibutylphthalate (DBP) and sodium tetra-phenylborate (Na-TPB) to the plasticized polyvinyl chloride matrices were prepared. The introduction of DBP in the membrane substantially increased the ability of both ionophores I and II to function as chromo ionophores. The advantages of the reported sensors include great stability, reproducibility, and relatively long lifespan, as well as excellent selectivity for Ni2+ ion detection across a wide range of alkali, alkaline earth, transition, and heavy metal ions.Under optimized membrane compositions and experimental parameters, the response of both sensors was linear throughout a concentration range of 3.5 × 10−8 to 8.1 × 10−5 and 2.0 × 10−8 to 5.1 × 10−5 M for I and II, respectively. Sensor detection and quantification limits based on the definition that the concentration of the sample leads to a signal equal to the blank signal plus three and ten times its standard deviation were determined to be 1.15 × 10−8 and 3.45 × 10−8 M when utilizing I, whereas they were 0.61 × 10−8 and 1.95 × 10−8 M when utilizing II, respectively. The reaction time of optodes is defined as the period required achieving 95% of based sensors and found to be 8.0 and 5.0 min using I and II, respectively. Ni2+ ion concentrations in water, food, and environmental samples were effectively determined using the proposed optical sensors.
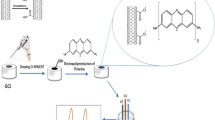
Graphical Abstract
Representative diagram for preparation of the sensing Ni2+ sensor








Similar content being viewed by others
Data availability
All data and materials should be available upon request.
References
Steinberg IM, Lobnik A, Wolfbeis OS. Characterisation of an optical sensor membrane based on the metal ion indicator pyrocatechol violet. Sens Actuators B. 2003;90:230–5. https://doi.org/10.1016/S0925-4005(03)00033-9.
Merian E Ed. Metals and their compounds in the environment, vol. 22, part 2. VCH, New York; 1991.
Cempel M, Nikel G. Nickel: a review of its sources and environmental toxicology. Pol J Environ Stud. 2006;15:75–382.
Bohn HL, McNeal BL, O’Connor GA. Soil chemistry. 2nd ed. Chichester: Wiley Interscience; 1985.
Frisbie SH, Mitchell EJ, Sarkar B. Urgent need to reevaluate the latest World Health Organization guidelines for toxic inorganic substances in drinking water. Environ Health. 2015;14:63–5. https://doi.org/10.1186/s12940-015-0050-7.
Mazloum M, Niassary MS, Amini MK. Pentacyclooctaaza as a neutral carrier in coated-wire ion-selective electrode for nickel(II). Sens Actuators B. 2002;82:259–64. https://doi.org/10.1016/S0925-4005(01)01017-6.
Repo E, Warchol JK, Kurniawan TA, Sillanpaa MET. Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: kinetic and equilibrium modeling. Chem Eng J. 2010;161:73–82. https://doi.org/10.1016/j.cej.2010.04.030.
Fu F, Chen R, Xiong Y. Comparative investigation of N, N-bis(dithio-carboxy)piperazine and diethyldithiocarbamate as precipitants for Ni(II) in simulated wastewater. J Hazard Mater. 2007;142:437–42. https://doi.org/10.1016/j.jhazmat.2006.08.036.
Amais RS, Ribeiro JS, Segatelli MG, Yoshida IVP, Luccas PO, et al. Assessment of nanocomposite alumina supported on multi-wall carbon nano-tubes as sorbent for on-line nickel preconcentration in water samples. Sep Purif Technol. 2007;58:122–8. https://doi.org/10.1016/j.seppur.2007.07.024.
Dadfarnia S, Shabani AMH, Bidabadi MS, Jafari AA. A novel ionic liquid/micro-volume back extraction procedure combined with flame atomic absorption spectrometry for determination of trace nickel in samples of nutritional interest. J Hazard Mater. 2010;173:534–8. https://doi.org/10.1016/j.jhazmat.2009.08.118.
Ahmadpour A, Tahmasbi M, Rohani Bastami T, AmelBesharati J. Rapid removal of cobalt ion from aqueous solutions by almond green hull. J Hazard Mat. 2009;166:925–30. https://doi.org/10.1016/j.jhazmat.2008.11.103.
Winiarski J, Popowicz E, Zdrojewicz Z. Nickel - role in human organism and toxic effects. Pol Med J XLI. 2016;241:115–8.
Guide lines for Drinking Water Quality. Health criteria and other supporting information, vol. 2, 2nd edn. World Health Organization, Geneva; 1998.
Alizadeh K, Nemati H, Zohrevand S, Hashemi P, Kakanejadifard A, Shamsipur M, Ganjali MR, Faridbod F. Selective dispersive liquid–liquid microextraction and preconcentration of Ni(II) into a micro droplet followed by ETAAS determination using a yellow Schiff’s base bisazanyl derivative. Mater Sci Eng C. 2013;33:916–22. https://doi.org/10.1016/j.msec.2012.11.021.
Gupta VK, Prasad R, Kumar P, Mangla R. New nickel(II) selective potentiometric sensor based on 5,7,12,14-tetramethyldibenzotetraaza-annulene in a poly(vinyl chloride) matrix. Anal Chim Acta. 2000;420:19–27. https://doi.org/10.1016/S0003-2670(00)01013-8.
Soares BM, Santos RF, Bolzan RC, Muller EI, Primela EG, Duarte FA. Simultaneous determination of iron and nickel in fluoropolymers by solid sampling high-resolution continuum source graphite furnace atomic absorption spectrometry. Talanta. 2016;160:454–60. https://doi.org/10.1016/j.talanta.2016.07.040.
Erulaş FA. Sensitive determination of nickel at trace levels in surface water samples by slotted quartz tube flame atomic absorption spectrometry after switchable solvent liquid-phase microextraction. Environ Monit Assess. 2020;192:272–8. https://doi.org/10.1007/s10661-020-8208-3.
Padilla V, Serrano N, Díaz-Cruz JM. Determination of trace levels of nickel(II) by adsorptive stripping voltammetry using a disposable and low-cost carbon screen-printed electrode. Chemosensors. 2021;9:94–105. https://doi.org/10.3390/chemosensors9050094.
Shinde AD, Acharya R, Reddy AVR. Analysis of zirconium and nickel based alloys and zirconium oxides by relative and internal mono-standard neutron activation analysis methods. Nucl Eng Technol. 2017;49:562–8. https://doi.org/10.1016/j.net.2016.09.009.
Pozzatti M, Nakadi FV, Rale MG, Welz B. Simultaneous determination of nickel and iron in vegetables of Solanaceae family using high-resolution continuum source graphite furnace atomic absorption spectrometry and direct solid sample analysis. Microchem J. 2017;133:162–7. https://doi.org/10.1016/j.microc.2017.03.021.
Soukal J, Musil S. Detailed evaluation of conditions of photochemical vapor generation for sensitive determination of nickel in water samples by ICP-MS detection. Microchem J. 2022;172:106963. https://doi.org/10.1016/j.microc.2021.106963.
Gab-Allah MA, Shehata AB. Determination of iron, nickel, and vanadium in crude oil by inductively coupled plasma optical emission spectrometry following microwave-assisted wet digestion. Chem Pap. 2021;75:4239–48. https://doi.org/10.1007/s11696-021-01633-8.
Ferreira VJ, Lemos VA, Teixeira LSG. Dynamic reversed-phase liquid-liquid microextraction for the determination of Cd, Cr, Mn, and Ni in vegetable oils by energy dispersive X-ray fluorescence spectrometry. J Food Compos Anal. 2023;117:105098. https://doi.org/10.1016/j.jfca.2022.105098.
Rehan I, Gondal MA, Rehan K, Sultana S, Khan S, Rehman MU, Waheed A, Salman SM. Nondestructive determination of chromium, nickel, and zinc in neem leaves and facial care products by laser induced breakdown spectroscopy (LIBS). Anal Lett. 2022;55:990–1003. https://doi.org/10.1080/00032719.2021.1979572.
Alharthi SS, Al-Saidi HM. Designing a simple semi-automated system for preconcentration and determination of nickel in some food samples using dispersive liquid–liquid microextraction based upon orange peel oil as extraction solvent. Arab J Chem. 2022;15:104094. https://doi.org/10.1016/j.arabjc.2022.104094.
Yang X, Yan C, Sun Y, Liu Y, Yang S, Deng Q, Wen X. Micro-spectrophotometric determination of nickel in Gentiana rigescens after switchable hydrophilicity solvent-based ultrasound-assisted liquid phase microextraction. Microchem J. 2021;168:106402. https://doi.org/10.1016/j.microc.2021.106402.
Bahrami M, Shabani AMH, Dadfarnia S, Moghadam MR, Baneshi M. Experimental design optimization of supramolecular dispersive liquid–liquid microextraction of nickel and its spectrophotometric determination. J Anal Chem. 2021;76:442–51. https://doi.org/10.1134/S106193482104002X.
Fendrych K, Porada R, Ba B. Electrochemical sensing platform based on Zeolite/Graphite/ Dimethylglyoxime nanocomposite for highly selective and ultrasensitive determination of nickel. J Hazard Mater. 2023;448:130953. https://doi.org/10.1016/j.jhazmat.2023.130953.
Rasolzadeh F, Hashemi P, Rezaei B. Aryl triazene derivative immobilized on agarose membrane for selective optical sensing and quantitation of Ni2+ in water. J Iran Chem Soc. 2019;16:1283–9. https://doi.org/10.1007/s13738-019-01603-8.
Jun-jie L, Chanf-jun H, Dan-qun H, Cai-hong S, Xiao-ganga L, Huan-baod F, Mei Y, JundaKey Z. Detection of trace nickel ions with a colorimetric sensor based on indicator displacement mechanism. Sens Actuators B. 2017;241:1294–302. https://doi.org/10.1016/j.snb.2016.09.191.
Alizadeh K, Rezaei B, Khazaeli E. An agarose based optical membrane sensor for selective monitoring of trace nickel ions. J Photochem Photobiol A. 2021;417:113371. https://doi.org/10.1016/j.jphotochem.2021.113371.
Suherman S, Hakim MS, Kuncaka A. Optical chemical sensor based on 2,2-furildioxime in sol-gel matrix for determination of Ni2+ in water. Processes. 2021;9:280–8. https://doi.org/10.3390/pr9020280.
Assiri MA, Junaid HM, Waseem MT, Hamad A, Shah SH, Iqbal J, Rauf W, Shahzad SA. AIEE active sensors for fluorescence enhancement based detection of Ni2+ in living cells: mechano-fluorochromic and photochromic properties with reversible sensing of acid and base. Anal Chim Acta. 2022;1234:340516. https://doi.org/10.1016/j.aca.2022.340516.
Iqbal J, Du Y, Howari F, Muhammad N, Rahim A. Simultaneous enrichment and on-line detection of low- concentration copper, cobalt, and nickel ions in water by near-infrared diffuse reflectance spectroscopy combined with chemometrics. J AOAC Int. 2017;100:560–5. https://doi.org/10.5740/jaoacint.16-0224.
Bodkhe GA, Khandagale DD, More MS, Deshmukh MA, Ingle NN, Sayyad PW, Mahadik MM, Shirsat SM, Al-Buriahi MS, Tsai M-L, Kim M, Shirsat MD. Ag@MOF-199 metal organic framework for selective detection of nickel ions in aqueous media. Ceramics Int. 2023;49:6772–9. https://doi.org/10.1016/j.ceramint.2022.10.135.
MohdDaniyal WMEM, Fen YW, Kamal Eddin FB, Abdullah J, Mahdi MA. Surface plasmon resonance assisted optical characterization of nickel ion solution and nanocrystalline cellulose-graphene oxide thin film for sensitivity enhancement analysis. Phys B. 2022;646:414292. https://doi.org/10.1016/j.physb.2022.414292.
Rossi A, Zannotti M, Cuccioloni M, Minicucci M, Petetta L, Angeletti M, Giovannetti R. Silver nanoparticle-based sensor for the selective detection of nickel ions. Nanomaterials. 2021;11:1733–48. https://doi.org/10.3390/nano11071733.
Rastegarzadeh S, Pourreza N, Saeedi I. An optical chemical sensor for thorium (IV) determination based on thorin. J Hazard Mater. 2010;173:110–4. https://doi.org/10.1016/j.jhazmat.2009.08.055.
Beiraghi A, Babaee S, Roshdi M. Spectrophotometric determination of trace amounts of beryllium using 1,8-dihydroxyanthrone as a new chromogenic reagent. J Hazard Mater. 2011;190:962–8. https://doi.org/10.1007/BF03247233.
Ensafi AA, Katiraeifar A, Meghdadi S. Highly selective optical-sensing film for lead (II) determination in water samples. J Hazard Mater. 2009;172:1069–75. https://doi.org/10.1016/j.jhazmat.2009.07.112.
Shamsipur M, Sadeghi M, Alizadeh K, Bencini A, Valtancoli B, et al. Novel fluorimetric bulk optode membrane based on 5,8-bis ((5-chloro-8-hydroxy-7-quinolinyl)methyl)-2,11-dithia-5,8-diaza-2,6-pyridinophane for selective detection of lead(II) ions. Talanta. 2010;80:2023–33. https://doi.org/10.1016/j.talanta.2009.11.011.
Amin AS. Optical chemical Sensor of lutetium(III) in water based on 2-nitro-6-(thiazol-2-yldiazenyl)phenol immobilized on polymethyl methacrylate and 2-nitrophenyl octyl ether matrix. Eurasian J Anal Chem. 2018;13:1–12.
Al-Mallah Z, Amin AS. Utilization of a triacetylcellulose membrane to immobilize 5-(2`,4`-dimethylphenylazo)-6-hydroxypyrimidine-2,4-dione for erbium determination in real sample. J Ind Eng Chem. 2018;63:281–7. https://doi.org/10.1016/j.jiec.2018.02.027.
Hassan N, Amin AS. Membrane optode for uranium(VI) preconcentration and colorimetric determination in real samples. RSC Adv. 2017;7:46566–74. https://doi.org/10.1039/C7RA08942B.
Badini GE, Grattan KTV, Tseung ACC. Impregnation of a pH-sensitive dye into sol-gels for fibre optic chemical sensors. Analyst. 1995;120:1025–8. https://doi.org/10.1039/AN9952001025.
Mahendra N, Gangaiya P, Sotheeswaran S, Narayanswamy R. Investigation of a Cu(II) fibre optic chemical sensor using fast sulphon black F (FSBF) immobilised onto XAD-7. Sens Actuators B. 2002;81:196–201. https://doi.org/10.1016/j.snb.2004.10.062.
Sanchez-Pedreno C, Garcıa MS, Ortuno JA, Albero MI, Ballester E. Development of a new flow-through bulk optode for the determination of manganese(II). Fresenius J Anal Chem. 2001;369:680–3. https://doi.org/10.1007/s002160100742.
Safavi A, Sadeghi M. Design and evaluation of a thorium (IV) selective optode. Anal Chim Acta. 2006;567:184–8. https://doi.org/10.1016/j.aca.2006.03.027.
Shamsipur M, Poursaberi T, Avanes A, Sharghi H. Copper(II)-selective fluorimetric bulk optode membrane based on a 1-hydroxy-9,10-anthraquinone derivative having two propenyl arms as a neutral fluorogenic ionophore. Spectrochim Acta A. 2006;63:43–8. https://doi.org/10.1016/j.saa.2005.04.013.
Sands TJ, Cardwell TJ, Cattrall RW, Farrell JR, Iles PJ, Kolev SD. A highly versatile stable optical sensor based on 4-decyloxy-2-(2-pyridylazo)-1-naphthol in Nafion for the determination of copper. Sens Actuators B. 2002;85:33–41. https://doi.org/10.1016/S0925-4005(02)00047-3.
Rouhollahi A, Shamsipur M. Triiodide PVC membrane electrode based on a charge-transfer complex of iodine with 2,4,6,8-tetraphenyl-2,4,6,8-tetraaza-bicyclo[3.3.0]octane. Anal Chem. 1999;71:1350–3. https://doi.org/10.1021/ac981077x.
Britton HTS. Hydrogen ions. 4th ed. London: Chapman and Hall; 1952.
Amin AS, Ahmed IS. Complexation and spectrophotometric study of samarium(III) using pyrimidine azo derivatives in the presence of cetyltri-methyl ammonium bromide. Anal Lett. 2010;43:2598–608. https://doi.org/10.1080/00032711003726910.
Amin AS. Determination of tin(IV) in alloys and in canned food by visible spectrophotometric technique using pyrimidine azo compounds in presence of mixed surfactants. Ann Chim. 2002;92:729–38.
Amin AS, Mohammed TY. Simultaneous spectrophotometric determination of thorium and rare earth metals with pyrimidine azo dyes and cetylpyridinium chloride. Talanta. 2001;54:611–6. https://doi.org/10.1016/S0039-9140(00)00679-2.
Amin AS, Mohammed TY, Mousa AA. Spectrophotometric studies and applications for the determination of yttrium in pure and in nickel base alloys. Spectrochim Acta B. 2003;59:2577–84. https://doi.org/10.1016/S1386-1425(03)00040-4.
ASTM Designation 4638, American Society for Testing and Materials, West Conshohocken, PA; 1995.
Shariff R, Aachary AA, Pacquette LH, Mittal AK, Girdhar R. Analytical method validation and determination of iron and phosphorus in vegetable oil by inductively coupled plasma–mass spectrometry with microwave assisted digestion. Anal Lett. 2018;51:1774–88. https://doi.org/10.1080/00032719.2017.1387554.
Oehme I, Prattes S, Wolfbeis OS, Mohr GJ. The effect of polymeric supports and methods of immobilization on the performance of an optical copper(II)-sensitive membrane based on the colorimetric reagent Zincon. Talanta. 1998;47:595–604. https://doi.org/10.1016/S0039-9140(98)00084-8.
Absalan G, Soleimani M, Asadi M, Ahmadi MB. Constructing a new optical sensor for monitoring ammonia in water samples using bis(acetylacetone ethylendiamine) tributylphosphin cobalt(III) tetraphenyl borate complex-coated triacetylcellulose. Anal Sci. 2004;20:1433–6. https://doi.org/10.2116/analsci.20.1433.
Uhrovčík J. Strategy for determination of LOD and LOQ values–Some basic aspects. Talanta. 2014;119:178–80. https://doi.org/10.1016/j.talanta.2013.10.061.
Kumar M, Rathore DPS, Singh AK. Amberlite XAD-2 functionalized with o-aminophenol: synthesis and applications as extractant for copper(II), cobalt(II), cadmium(II), nickel(II), zinc(II) and lead(II). Talanta. 2000;51:1187–96. https://doi.org/10.1016/S0039-9140(00)00295-2.
Ferreira SLC, dos Santosa WNL, Lemos VA. On-line preconcentration system for nickel determination in food samples by flame atomic absorption spectrometry. Anal Chim Acta. 2001;445:145–51. https://doi.org/10.1016/S0003-2670(01)01262-4.
Narin I, Soylak M. The uses of 1-(2-pyridylazo) 2-naphtol (PAN) impregnated Ambersorb 563 resin on the solid phase extraction of traces heavy metal ions and their determinations by atomic absorption spectrometry. Talanta. 2003;60:215–22. https://doi.org/10.1016/S0039-9140(03)00039-0.
Kenduzler E, Baytak S, Yalcınkaya O, Turker AR. Application of factorial design in optimization of nickel preconcentration by solid phase extraction and its determination in water and food samples by flame atomic absorption spectrometry. Can J Anal Sci Spect. 2007;52:91–94. https://hdl.net/20.500.12513/1873.
Arpa C, Bektas S. Preconcentration and determination of lead, cadmium and nickel from water samples using a polyethylene glycol dye immobilized on poly (hydroxyethylmethacrylate) microspheres. Anal Sci. 2006;22:1025–9. https://doi.org/10.2116/analsci.22.1025.
Ghaedi M, Ahmadi F, Shokrollahi A. Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry. J Hazard Mater. 2007;142:272–8. https://doi.org/10.1016/j.jhazmat.2006.08.012.
Ciftci H, Yalcin H, Eren E, Olcucu A, Sekerci M. Enrichment and determination of Ni2+ ions in water samples with a diamino-4-(4-nitro-phenylazo)-1H-pyrazole (PDANP) by using FAAS. Desalination. 2010;256:48–53. https://doi.org/10.1016/j.desal.2010.02.018.
Praveen RS, Daniel S, Rao TP. Solid phase extraction preconcentration of cobalt and nickel with 5, 7-dichloroquinone-8-ol embedded styrene–ethylene glycol dimethacrylate polymer particles and determination by flame atomic absorption spectrometry (FAAS). Talanta. 2005;66:513–20. https://doi.org/10.1016/j.talanta.2004.11.026.
Rezaei B, Hadadzadeh H, Azimi A. Nickel(II) selective PVC-based membrane sensor using a Schiff base. Int J Spect. 2011;2011:1–7. https://doi.org/10.1155/2011/746372.
Amini MK, Momeni-Isfahani T, Khorasani JH, Pourhossein M. Development of an optical chemical sensor based on 2-(5-bromo-2-pyridylazo)-5-(diethylamino) phenol in Nafion for determination of nickel ion. Talanta. 2004;63:713–20. https://doi.org/10.1016/j.talanta.2003.12.032.
Ensafi AA, Bakhshi M. New stable optical film sensor based on immobilization of 2-amino-1-cyclopentene-1-dithiocarboxylic acid on acetyl cellulose membrane for Ni(II) determination. Sens Actuators B. 2003;96:435–40. https://doi.org/10.1016/j.talanta.2003.12.032.
Hashemi P, Hosseini M, Zargoosh K, Alizadeh K. High sensitive optode for selective determination of Ni2+ based on the covalently immobilized thionine in agarose membrane. Sens Actuators B. 2011;153:24–8. https://doi.org/10.1016/j.snb.2010.09.068.
Shamsipur M, Poursaberi T, Karami AR, Hosseini M, Momeni A, Alizadeh N, Yousefi M, Ganjali MR. Development of a new fluorimetric bulk optode membrane based on 2,5-thiophenylbis(5-tert-butyl-1,3-benzexazole) for nickel(II) ions. Anal Chim Acta. 2004;501:55–60. https://doi.org/10.1016/j.aca.2003.09.008.
Babaee S, Pakdehi SG, Nabavi AS. An optical chemical sensor for determination of nickel in water and hydrogen peroxide samples. J New Develop Chem. 2016;1:58–69. https://doi.org/10.14302/issn.2377-2549.jndc-16-1212.
Alizadeh K, Rezaei B, Khazaeli E. A new triazene-1-oxide derivative, immobilized on the triacetylcellulose membrane as an optical Ni2+ sensor. Sens Actuators B. 2014;193:267–72. https://doi.org/10.1016/j.snb.2013.11.061.
Yoshikuni N, Baba T, Tsunoda N, Oguma K. Aqueous two-phase extraction of nickel dimethylglyoximato complex and its application to spectrophotometric determination of nickel in stainless steel. Talanta. 2005;66:40–4. https://doi.org/10.1016/j.talanta.2004.09.014.
Desai VK, Desai KK. 2-Hydroxy-4-isopropoxyacetophenone thiosemi-carbazone as a spectrophotometric reagent for nickel(II). Orient J Chem. 1996;12:203–14.
Otomo M, Watanabe T, Moriya M. Solvent extraction and spectrophoto-metric determination of nickel(II) with thiazole-2-carbaldehyde 2-quinolyl- hydrazone. Anal Sci. 1986;2:549–52. https://doi.org/10.2116/analsci.2.549.
Jadhav VA, Kulkarni MU. 7-Methyl-2-chloroquinoline-3-carbaldehyde thiosemicarbazone as analytical reagent for copper,-cobalt- and nickel (II). J Indian Chem Soc. 1992;69:287–9.
Reddy AV, Reddy YK. Sequential extraction and determination of copper and nickel with 2,4-dihydroxyacetophenone thiosemicarbazone, sequential extraction and determination of copper and nickel with 2,4-dihydroxy-acetophenone thiosemicarbazone. Talanta. 1986;33:617–9. https://doi.org/10.1016/0039-9140(86)80141-2.
Ramachandraiah C, Kumar JR, Reddy KJ, Narayana SL, Reddy AV. Development of a highly sensitive extractive spectrophotometric method for the determination of nickel(II) from environmental matrices using N-ethyl-3-carbazolecarboxaldehyde-3-thiosemicarbazone. J Environ Manag. 2008;88:729–36. https://doi.org/10.1016/j.jenvman.2007.03.033.
Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry. 5th ed. London: Prentice-Hall; 2005.
Acknowledgements
The authors would like to acknowledge the financial support from the Department of Chemistry, Faculty of Science, Benha, Port Said, and Taibah Universities, for providing instrumental facilities.
Author information
Authors and Affiliations
Contributions
Reem F. Alshehri and Eman R. Darwish supplied analyzed, collected, designed interpreted data and wrote the paper. The paper has been read and approved by all authors. Alaa S. Amin conceived and designed the work and wrote the paper.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflicts of interests
No potential conflict of interest was reported by the author(s).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alshehri, R.F., Amin, A.S. & Darwish, E.R. Ultrasensitive and highly selective detection of nickel ion by two novel optical sensors. Anal Bioanal Chem 415, 5695–5707 (2023). https://doi.org/10.1007/s00216-023-04845-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04845-x