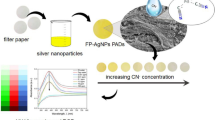
Abstract
A colorimetric paper-based enzyme-coupled antimony tin oxide nanoparticle (ATONP) nanobiosensor for selective detection of Cd2+ ions in clams and mussels is presented. Alkaline phosphatase (ALP) was immobilized on ATONPs via 16-phosphonohexadecanoic acid (16-PHA) to develop ATONP-ALP nanobiosensor. The biosensor was characterized using XPS, Raman spectroscopy, SEM, and EDX. ATONP-ALP nanobiosensor exhibited high selectivity towards detection of Cd2+ ion with a LOD 0.006 μg L−1 and linear range of detection 0.005–1 μg L−1. The developed biosensor was further integrated into a low-cost paper-based format. A visual color change was obtained for Cd2+ ion in the range 0.1–10 μg L−1. The developed biosensor was successfully demonstrated for the analysis of Cd2+ ions in clams with recoveries 101–104%. The ATONP-ALP nanobiosensor was validated using mussel tissue (BCR-668) and the conventional ICP-OES and ICP-MS techniques.
Graphical abstract






Similar content being viewed by others
References
Reimann C, Fabian K, Flem B. Cadmium enrichment in topsoil: separating diffuse contamination from biosphere-circulation signals. Sci Total Environ. 2019;651:1344–55.
Mezynska M, Brzóska MM. Environmental exposure to cadmium—a risk for health of the general population in industrialized countries and preventive strategies. Environ Sci Pollut Res. 2018;25(4):3211–32.
Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. 1988;333(6169):134–9.
Dharma-wardana MWC. Fertilizer usage and cadmium in soils, crops and food. Environ Geochem Health. 2018;40(6):2739–59.
Simpson W. A critical review of cadmium in the marine environment. Prog Oceanogr. 1981;10(1):1–70.
Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010;118(2):182–90.
Nishijo M, Nakagawa H. Effects of cadmium exposure on life prognosis. Cadmium Toxicity. Singapore: Springer; 2019. p. 63–73.
Waalkes MP. Cadmium carcinogenesis in review. J Inorg Biochem. 2000;79(1–4):241–4.
Wang C, Sheng J, Hong Y, Peng K, Wang J, Wu D, et al. Molecular characterization and expression of metallothionein from freshwater pearl mussel, Hyriopsis schlegelii. Biosci Biotechnol Biochem. 2016;80(7):1327–35.
Couillard Y, Campbell PGC, Tessier A. Response of metallothionein concentrations in a freshwater bivalve (Anodonta grandis) along an environmental cadmium gradient. Limnol Oceanogr. 1993;38(2):299–313.
JECFA, Joint FAO/WHO. Food Standards Programme Codex Alimentarius Commission, Report of the 38th session of the Codex Committee on Food Additives and Contaminants, LINORM 06/12A. 2006.
FSSAI, Food Safety, and Standards (Contaminants, Toxins, and Residues) Regulations 2011, Notification No. 2-15015/30/2010.
Li L, Hu B, Xia L, Jiang Z. Determination of trace Cd and Pb in environmental and biological samples by ETV-ICP-MS after single-drop microextraction. Talanta. 2006;70(2):468–73.
Soylak M, Kars A, Narin I. Coprecipitation of Ni2+, Cd2+ and Pb2+ for preconcentration in environmental samples prior to flame atomic absorption spectrometric determinations. J Hazard Mater. 2008;159(2):435–9.
Suleiman JS, Hu B, Huang C, Zhang N. Determination of Cd, Co, Ni and Pb in biological samples by microcolumn packed with black stone (Pierre noire) online coupled with ICP-OES. J Hazard Mater. 2008;157(2):410–7.
Chen W, Fang X, Li H, Cao H, Kong J. A simple paper-based colorimetric device for rapid mercury (II) assay. Sci Rep. 2016;6(1):31948.
Cate DM, Dungchai W, Cunningham JC, Volckens J, Henry CS. Simple, distance-based measurement for paper analytical devices. Lab Chip. 2013;13(12):2397–404.
Yan J, Ge L, Song X, Yan M, Ge S, Yu J. Paper-based electrochemiluminescent 3D immunodevice for lab-on-paper, specific, and sensitive point-of-care testing. Chem Eur J. 2012;18(16):4938–45.
He M, Liu Z. Based microfluidic device with upconversion fluorescence assay. Anal Chem. 2013;85(24):11691–4.
Ge L, Yan J, Song X, Yan M, Ge S, Yu J. Three-dimensional paper-based electrochemiluminescence immunodevice for multiplexed measurement of biomarkers and point-of-care testing. Biomaterials. 2012;33(4):1024–31.
Cheng CM, Martinez AW, Gong J, Mace CR, Phillips ST, Carrilho E, et al. Paper-based ELISA. Angew Chem Int Ed. 2010;49(28):4771–4.
Lopez-Ruiz N, Curto VF, Erenas MM, Benito-Lopez F, Diamond D, Palma AJ, et al. Smartphone-based simultaneous pH and nitrite colorimetric determination for paper microfluidic devices. Anal Chem. 2014;86(19):9554–62.
Sener G, Uzun L, Denizli A. Colorimetric sensor array based on gold nanoparticles and amino acids for identification of toxic metal ions in water. ACS Appl Mater Interfaces. 2014;6(21):18395–400.
Homaei A. Immobilization of Penaeus merguiensis alkaline phosphatase on gold nanorods for heavy metal detection. Ecotoxicol Environ Saf. 2017;136:1–7.
Lei C, Dai H, Fu Y, Ying Y, Li Y. Colorimetric sensor array for thiols discrimination based on urease–metal ion pairs. Anal Chem. 2016;88(17):8542–7.
Tang Z, Chen H, He H, Ma C. Assays for alkaline phosphatase activity: progress and prospects. Trends Anal Chem. 2019;113:32–43.
Aldewachi H, Chalati T, Woodroofe M, Bricklebank N, Sharrack B, Gardiner P. Gold nanoparticle-based colorimetric biosensors. Nanoscale. 2018;10(1):18–33.
Shi X, Gu W, Li B, Chen N, Zhao K, Xian Y. Enzymatic biosensors based on the use of metal oxide nanoparticles. Microchim Acta. 2014;181(1–2):1–22.
Rahman MM, Ahmed J, Asiri AM. Development of creatine sensor based on antimony-doped tin oxide (ATO) nanoparticles. Sensors Actuators B Chem. 2017;242:167–75.
Wang Z, Wang K, Zhao L, Chai S, Zhang J, Zhang X, et al. A novel sensor made of antimony doped tin oxide-silica composite sol on a glassy carbon electrode modified by single-walled carbon nanotubes for detection of norepinephrine. Mater Sci Eng C. 2017;80:180–6.
Li Y, Sun J, Mao W, Tang S, Liu K, Qi T, et al. Antimony-doped tin oxide nanoparticles as peroxidase mimics for paper-based colorimetric detection of glucose using smartphone read-out. Microchim Acta. 2019;186(7):403.
Pal S, Bhand S. Zinc oxide nanoparticle-enhanced ultrasensitive chemiluminescence immunoassay for the carcinoma embryonic antigen. Microchim Acta. 2015;182(9–10):1643–51.
Krishnakumar T, Jayaprakash R, Pinna N, Phani AR, Passacantando M, Santucci S. Structural, optical and electrical characterization of antimony-substituted tin oxide nanoparticles. J Phys Chem Solids. 2009;70(6):993–9.
Müller V, Rasp M, Štefanić G, Ba J, Günther S, Rathousky J, et al. Highly conducting nanosized monodispersed antimony-doped tin oxide particles synthesized via nonaqueous sol−gel procedure. Chem Mater. 2009;21(21):5229–36.
Kuck JFR, Yu N-T, Askren CC. Total sulfhydryl by Raman spectroscopy in the intact lens of several species: variations in the nucleus and along the optical axis during aging. Exp Eye Res. 1982;34(1):23–37.
Swain KK, Balasubramaniam R, Bhand S. A portable microfluidic device-based Fe3O4–urease nanoprobe-enhanced colorimetric sensor for the detection of heavy metals in fish tissue. Prep Biochem Biotechnol. 2020;50:1000–13.
Treviño S, Andrade-García A, Herrera Camacho I, León-Chavez BA, Aguilar-Alonso P, Flores G, et al. Chronic cadmium exposure lead to inhibition of serum and hepatic alkaline phosphatase activity in Wistar rats. J Biochem Mol Toxicol. 2015;29(12):587–94.
Du J, Hu X, Zhang G, Wu X, Gong D. Colorimetric detection of cadmium in water using L-cysteine functionalized gold–silver nanoparticles. Anal Lett. 2018;51(18):2906–19.
Song S, Zou S, Zhu J, Liu L, Kuang H. Immunochromatographic paper sensor for ultrasensitive colorimetric detection of cadmium. Food Agric Immunol. 2018;29(1):3–13.
Luan Y, Lu A, Chen J, Fu H, Xu L. A label-free aptamer-based fluorescent assay for cadmium detection. Appl Sci. 2016;6(12):432.
Gan Y, Liang T, Hu Q, Zhong L, Wang X, Wan H, et al. In-situ detection of cadmium with aptamer functionalized gold nanoparticles based on smartphone-based colorimetric system. Talanta. 2020;208:1202–31.
Silwana B, Van Der Horst C, Iwuoha E, Somerset V. Amperometric determination of cadmium, lead, and mercury metal ions using a novel polymer immobilised horseradish peroxidase biosensor system. J Environ Sci Health A. 2014;49(13):1501–11.
Meredith NA, Volckens J, Henry CS. Based microfluidics for experimental design: screening masking agents for simultaneous determination of Mn (II) and Co (II). Anal Lett. 2017;9(3):534–40.
Xiao N, Dong JX, Liu SG, Li N, Fan YZ, Ju YJ, et al. Multifunctional fluorescent sensors for independent detection of multiple metal ions based on Ag nanoclusters. Sensors Actuators B Chem. 2018;264:184–92.
Acknowledgments
The authors acknowledge the CSIF Facility of BITS Goa and Hyderabad Campuses.
Funding
Sunil Bhand acknowledges DAE-BRNS, India, for funding the project. Krishna Kumari Swain acknowledges BITS Pilani K.K. Birla Goa Campus for providing the fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research was conducted as per the Indian ethical guidelines for seafood.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 4.97 kb).
Rights and permissions
About this article
Cite this article
Swain, K.K., Bhand, S. A colorimetric paper-based ATONP-ALP nanobiosensor for selective detection of Cd2+ ions in clams and mussels. Anal Bioanal Chem 413, 1715–1727 (2021). https://doi.org/10.1007/s00216-020-03140-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-03140-3