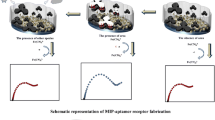
Abstract
The polymerization of norepinephrine, and the properties of the related polymer polynorepinephrine, started to be investigated barely 9 years ago and only few works were produced so far, mainly in materials science and medicine. An unexpectedly low relevance, especially if compared with the interest toward dopamine and polydopamine, differing from norepinephrine only for a hydroxyl group and whose properties were deeply investigated and applied to an impressive number of subject areas. We show here that in some cases, norepinephrine and dopamine monomers can be exchanged without virtually affecting the experimental results. But even more interesting, the choice of norepinephrine can positively influence the properties of the final polymer. In particular, the smoother and more hydrophilic surface of polynorepinephrine may enhance cell adhesion and proliferation, increase the activity of conjugated biomolecules, and induce higher cellular uptake of nanodrugs. Moreover, polynorepinephrine presents an additional anchoring point that can be exploited for further functionalization. Nevertheless, despite its potential for bioconjugation and molecular recognition, polynorepinephrine has not yet been considered in biosensing. Here we report our feelings in terms of perspective use of polynorepinephrine as new functional monomer for biomimetic receptor development by molecular imprinting, with application in affinity biosensing.

Graphical abstracts




Similar content being viewed by others
References
Blows WT. Neurotransmitters of the brain: serotonin, noradrenaline (Norepinephrine) and dopamine. J Neurosci Nurs. 2000;32:234–8. https://doi.org/10.1097/01376517-200008000-00008.
Barclay TG, Hegab HM, Clarke SR, Ginic-Markovic M. Versatile surface modification using polydopamine and related polycatecholamines: chemistry, structure, and applications. Adv Mater Interfaces. 2017;4:1–38. https://doi.org/10.1002/admi.201601192.
Liu M, Zeng G, Wang K, Wan Q, Tao L, Zhang X, et al. Recent developments in polydopamine: an emerging soft matter for surface modification and biomedical applications. Nanoscale. 2016;8:16819–40. https://doi.org/10.1039/c5nr09078d.
Lynge ME, Van Der Westen R, Postma A, Städler B. Polydopamine - a nature-inspired polymer coating for biomedical science. Nanoscale. 2011;3:4916–28. https://doi.org/10.1039/C1NR10969C.
Scarano S, Pascale E, Palladino P, Fratini E, Minunni M. Determination of fermentable sugars in beer wort by gold nanoparticles@polydopamine: a layer-by-layer approach for localized surface plasmon resonance measurements at fixed wavelength. Talanta. 2018;183:24–32. https://doi.org/10.1016/j.talanta.2018.02.044.
Scarano S, Palladino P, Pascale E, Brittoli A, Minunni M. Colorimetric determination of p-nitrophenol by using ELISA microwells modified with an adhesive polydopamine nanofilm containing catalytically active gold nanoparticles. Microchim Acta. 2019;186:146. https://doi.org/10.1007/s00604-019-3259-2.
Kim S, Lee S, Park J, Lee JY. Electrochemical co-deposition of polydopamine/hyaluronic acid for anti-biofouling bioelectrodes. Front Chem. 2019;7:262. https://doi.org/10.3389/fchem.2019.00262.
Ding YH, Floren M, Tan W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurface and Biotribology. 2016;2:121–36. https://doi.org/10.1016/j.bsbt.2016.11.001.
Zaidi SA. An account on the versatility of dopamine as a functional monomer in molecular imprinting. Chem Sel. 2019;4:5081–90. https://doi.org/10.1002/slct.201901029.
Palladino P, Bettazzi F, Scarano S. Polydopamine: surface coating, molecular imprinting, and electrochemistry — successful applications and future perspectives in (bio)analysis. Anal Bioanal Chem. 2019;411:4327–38. https://doi.org/10.1007/s00216-019-01665-w.
Palladino P, Brittoli A, Pascale E, Minunni M, Scarano S. Colorimetric determination of total protein content in serum based on the polydopamine/protein adsorption competition on microplates. Talanta. 2019;198:15–22. https://doi.org/10.1016/j.talanta.2019.01.095.
Palladino P, Minunni M, Scarano S. Cardiac troponin T capture and detection in real-time via epitope-imprinted polymer and optical biosensing. Biosens Bioelectron. 2018;106:93–8. https://doi.org/10.1016/j.bios.2018.01.068.
Jeon YJ, Kang SM. Chemically stable poly(norepinephrine) coatings on solid substrates by post-oxidation. Polym Degrad Stab. 2013;98:1271–3. https://doi.org/10.1016/j.polymdegradstab.2013.03.013.
Hong S, Kim J, Na YS, Park J, Kim S, Singha K, et al. Poly(norepinephrine): ultrasmooth material-independent surface chemistry and nanodepot for nitric oxide. Angew Chemie - Int Ed. 2013;52:9187–91. https://doi.org/10.1002/anie.201301646.
Kang SM, Rho J, Choi IS, Messersmith PB, Lee H. Norepinephrine: material-independent, multifunctional surface modification reagent. J Am Chem Soc. 2009;131:13224–5. https://doi.org/10.1021/ja905183k.
Kang SM, Lee H. One-step immobilization of initiators for surface-initiated ring opening polymerization and atom transfer radical polymerization by poly(norepinephrine) coating. J Nanosci Nanotechnol. 2015;15:1597–600. https://doi.org/10.1166/jnn.2015.9284.
Son EJ, Kim JH, Kim K, Park CB. Quinone and its derivatives for energy harvesting and storage materials. J Mater Chem A. 2016;4:11179–202. https://doi.org/10.1039/C6TA03123D.
Jung S, Kim H, Lee J, Jeong G, Kim H, Park J, et al. Bio-inspired catecholamine-derived surface modifier for graphene-based organic solar cells. ACS Appl Energy Mater. 2018;1:6463–8. https://doi.org/10.1021/acsaem.8b01396.
Kang SM, Park S, Kim D, Park SY, Ruoff RS, Lee H. Simultaneous reduction and surface functionalization of graphene oxide by mussel-inspired chemistry. Adv Funct Mater. 2011;21:108–12. https://doi.org/10.1002/adfm.201001692.
Hong JY, Yu X, Bak BM, Pang C, Park HS. Bio-inspired functionalization and redox charge transfer of graphene oxide sponges for pseudocapacitive electrodes. Carbon. 2015;83:71–8. https://doi.org/10.1016/j.carbon.2014.11.020.
Son EJ, Kim JH, Ko JW, Park CB. Catecholamine-functionalized graphene as a biomimetic redox shuttle for solar water oxidation. Faraday Discuss. 2017;198:135–45. https://doi.org/10.1039/C6FD00190D.
Lee W, Lee JU, Byun JH. Catecholamine polymers as surface modifiers for enhancing interfacial strength of fiber-reinforced composites. Compos Sci Technol. 2015;110:53–61. https://doi.org/10.1016/j.compscitech.2015.01.021.
Chwatko M, Arena JT, McCutcheon JR. Norepinephrine modified thin film composite membranes for forward osmosis. Desalination. 2017;423:157–64. https://doi.org/10.1016/j.desal.2017.07.025.
Xia L, Vemuri B, Gadhamshetty V, Kilduff J. Poly (ether sulfone) membrane surface modification using norepinephrine to mitigate fouling. J Memb Sci. 2019; Article in press. 117657. https://doi.org/10.1016/j.memsci.2019.117657.
Iwasaki T, Tamai Y, Yamamoto M, Taniguchi T, Kishikawa K, Kohri M. Melanin precursor influence on structural colors from artificial melanin particles: PolyDOPA, polydopamine, and polynorepinephrine. Langmuir. 2018;34:11814–21. https://doi.org/10.1021/acs.langmuir.8b02444.
Kang SM, Lee H. Surface modification of highly ordered pyrolytic graphite (HOPG) by a mussel-inspired poly(norepinephrine) coating: characterizations and cell adhesion test. Bull Kor Chem Soc. 2013;34:960–2. https://doi.org/10.5012/bkcs.2013.34.3.960.
Park M, Shin M, Kim E, Lee S, Park KI, Lee H, et al. The promotion of human neural stem cells adhesion using bioinspired poly(norepinephrine) nanoscale coating. J Nanomater. 2014;2014:1–10. https://doi.org/10.1155/2014/793052.
Taskin MB, Xu R, Zhao H, Wang X, Dong M, Besenbacher F, et al. Poly(norepinephrine) as a functional bio-interface for neuronal differentiation on electrospun fibers. Phys Chem Chem Phys. 2015;17:9446–53. https://doi.org/10.1039/c5cp00413f.
Jiang X, Li Y, Liu Y, Chen C, Chen M. Selective enhancement of human stem cell proliferation by mussel inspired surface coating. RSC Adv. 2016;6:60206–14. https://doi.org/10.1039/c6ra11173d.
Liu Y, Zhou G, Liu Z, Guo M, Jiang X, Taskin MB, et al. Mussel inspired polynorepinephrine functionalized electrospun polycaprolactone microfibers for muscle regeneration. Sci Rep. 2017;7:8197. https://doi.org/10.1038/s41598-017-08572-z.
Toita R, Sunarso, Rashid AN, Tsuru K, Ishikawa K. Modulation of the osteoconductive property and immune response of poly(ether ether ketone) by modification with calcium ions. J Mater Chem B 2015;3:8738–8746. https://doi.org/10.1039/C5TB01679G.
Li L, Li Y, Yang L, Yu F, Zhang K, Jin J, et al. Polydopamine coating promotes early osteogenesis in 3D printing porous Ti6Al4V scaffolds. Ann Transl Med. 2019;7:240. https://doi.org/10.21037/atm.2019.04.79.
Chen Y, Taskin MB, Zhang Z, Su Y, Han X, Chen M. Bioadhesive anisotropic nanogrooved microfibers directing three-dimensional neurite extension. Biomater Sci. 2019;7:2165–73. https://doi.org/10.1039/C8BM01603H.
Chen X, Cortez-Jugo C, Choi GH, Björnmalm M, Dai Y, Yoo PJ, et al. Patterned poly(dopamine) films for enhanced cell adhesion. Bioconjug Chem. 2017;28:75–80. https://doi.org/10.1021/acs.bioconjchem.6b00544.
Hong D, Lee H, Ko EH, Lee J, Cho H, Park M, et al. Organic/inorganic double-layered shells for multiple cytoprotection of individual living cells. Chem Sci. 2015;6:203–8. https://doi.org/10.1039/C4SC02789B.
Kim E, Lee S, Hong S, Jin G, Kim M, Park KI, et al. Sticky “delivering-from” strategies using viral vectors for efficient human neural stem cell infection by bioinspired catecholamines. ACS Appl Mater Interfaces. 2014;6:8288–94. https://doi.org/10.1021/am5011095.
Liu X, Xie Z, Shi W, He Z, Liu Y, Su H, et al. Polynorepinephrine nanoparticles: a novel photothermal nanoagent for chemo-photothermal cancer therapy. ACS Appl Mater Interfaces. 2019;11:19763–73. https://doi.org/10.1021/acsami.9b03458.
He Z, Su H, Shen Y, Shi W, Liu X, Liu Y, et al. Poly(norepinephrine)-coated FeOOH nanoparticles as carriers of artemisinin for cancer photothermal-chemical combination therapy. RSC Adv. 2019;9:9968–82. https://doi.org/10.1039/c9ra01289c.
Luo D, Carter KA, Miranda D, Lovell JF. Chemophototherapy: an emerging treatment option for solid tumors. Adv Sci. 2017;4:1600106. https://doi.org/10.1002/advs.201600106.
Chen J, Liang RP, Wang XN, Qiu JD. A norepinephrine coated magnetic molecularly imprinted polymer for simultaneous multiple chiral recognition. J Chromatogr A. 2015;1409:268–76. https://doi.org/10.1016/j.chroma.2015.07.052.
Liang RP, Xiang CY, Wang JW, Qiu JD. Preparation of polynorepinephrine adhesive coating via one-step self-polymerization for enantioselective capillary electrochromatography coupled with electrogenerated chemiluminesense detection. J Chromatogr A. 2013;1284:194–201. https://doi.org/10.1016/j.chroma.2013.02.007.
Xiao X, Zhang Y, Wu J, Jia L. Poly(norepinephrine)-coated open tubular column for the separation of proteins and recombination human erythropoietin by capillary electrochromatography. J Sep Sci. 2017;40:4636–44. https://doi.org/10.1002/jssc.201700720.
Chen J, Liang RP, Wu LL, Qiu JD. One-step preparation and application of mussel-inspired poly(norepinephrine)-coated polydimethylsiloxane microchip for separation of chiral compounds. Electrophoresis. 2016;37:1676–84. https://doi.org/10.1002/elps.201600054.
Wu J, Xiao X, Li Z, Jia L. Enantioseparation of chiral Β-blockers using polynorepinephrine-coated nanoparticles and chiral capillary electrophoresis. Anal Bioanal Chem. 2019;411:2121–9. https://doi.org/10.1007/s00216-019-01641-4.
Qiu J, Chen G, Liu S, Zhang T, Wu J, Wang F, et al. Bioinspired polyelectrolyte-assembled graphene-oxide-coated C18 composite solid-phase microextraction fibers for in vivo monitoring of acidic pharmaceuticals in fish. Anal Chem. 2016;88:5841–8. https://doi.org/10.1021/acs.analchem.6b00417.
Qiu J, Chen G, Zhu F, Ouyang G. Sulfonated nanoparticles doped electrospun fibers with bioinspired polynorepinephrine sheath for in vivo solid-phase microextraction of pharmaceuticals in fish and vegetable. J Chromatogr A. 2016;1455:20–7. https://doi.org/10.1016/j.chroma.2016.05.082.
Jiang Y, Wang Y, Wang H, Zhou L, Gao J, Zhang Y, et al. Facile immobilization of enzyme on three dimensionally ordered macroporous silica via a biomimetic coating. New J Chem. 2015;39:978–84. https://doi.org/10.1039/C4NJ01947D.
Yang D, Wang X, Ai Q, Shi J, Jiang Z. Performance comparison of immobilized enzyme on the titanate nanotube surfaces modified by poly(dopamine) and poly(norepinephrine). RSC Adv. 2015;5:42461–7. https://doi.org/10.1039/C5RA02420J.
Liu Y, Nan X, Shi W, Liu X, He Z, Sun Y, et al. A glucose biosensor based on the immobilization of glucose oxidase and Au nanocomposites with polynorepinephrine. RSC Adv. 2019;9:16439–46. https://doi.org/10.1039/c9ra02054c.
Khetani S, Kollath VO, Eastick E, Debert C, Sen A, Karan K, et al. Single-step functionalization of poly-catecholamine nanofilms for ultra-sensitive immunosensing of ubiquitin carboxyl terminal hydrolase-L1 (UCHL-1) in spinal cord injury. Biosens Bioelectron. 2019;145:111715. https://doi.org/10.1016/j.bios.2019.111715.
Farahmand Nejad MA, Ghasemi F, Hormozi-nezhad MR. A wide-color-varying ratiometric nanoprobe for detection of norepinephrine in urine samples. Anal Chim Acta. 2018;1039:124–31. https://doi.org/10.1016/j.aca.2018.07.043.
Accardo A, Morisco A, Palladino P, Palumbo R, Tesauro D, Morelli G. Amphiphilic CCK peptides assembled in supramolecular aggregates: structural investigations and in vitro studies. Mol BioSyst. 2011;7:862–70. https://doi.org/10.1039/c0mb00238k.
Palladino P, Castelletto V, Dehsorkhi A, Stetsenko D, Hamley IW. Reversible thermal transition of polydiacetylene based on KTTKS collagen sequence. Chem Commun. 2012;48:9774–6. https://doi.org/10.1039/c2cc34665f.
Palladino P, Castelletto V, Dehsorkhi A, Stetsenko D, Hamley IW. Conformation and self-association of peptide amphiphiles based on the KTTKS collagen sequence. Langmuir. 2012;28:12209–15. https://doi.org/10.1021/la302123h.
Diaferia C, Gianolio E, Palladino P, Arena F, Boffa C, Morelli G, et al. Peptide materials obtained by aggregation of polyphenylalanine conjugates as gadolinium-based magnetic resonance imaging contrast agents. Adv Funct Mater. 2015;25:7003–16. https://doi.org/10.1002/adfm.201502458.
Baldoneschi V, Palladino P, Banchini M, Minunni M, Scarano S. Norepinephrine as new functional monomer for molecular imprinting: an applicative study for the optical sensing of cardiac biomarkers. Biosens. Bioelectron. under revision.
Palladino P, Scaglione GL, Arcovito A, Vitale RM, Amodeo P, Vallone B, et al. Neuroglobin-prion protein interaction: What’s the function? J Pept Sci. 2011;17:387–91. https://doi.org/10.1002/psc.1333.
Palladino P, Aura AM, Spoto G. Surface plasmon resonance for the label-free detection of Alzheimer’s β-amyloid peptide aggregation. Anal Bioanal Chem. 2016;408:849–54. https://doi.org/10.1007/s00216-015-9172-6.
D’Agata R, Palladino P, Spoto G. Streptavidin-coated gold nanoparticles: critical role of oligonucleotides on stability and fractal aggregation. Beilstein J Nanotechnol. 2017;8:1–11. https://doi.org/10.3762/bjnano.8.1.
Torrini F, Palladino P, Brittoli A, Baldoneschi V, Minunni M, Scarano S. Characterization of troponin T binding aptamers for an innovative enzyme-linked oligonucleotide assay (ELONA). Anal Bioanal Chem. 2019;411:7709–16. https://doi.org/10.1007/s00216-019-02014-7.
Funding
The authors thank the following projects for the financial support: Ministry of Education, University and Research (MIUR), through the Project “Dipartimenti di Eccellenza 2018-2022”; the Italian Ministry of Health for the project “Development of optical biosensors for the detection of peptide hormones through molecularly imprinted polymers” within the 2018 call “Research and training/information program on drugs, medical substances and practices that can be used for doping purposes and for health protection in sporting activities”; the Tuscany Region within the POR-FESR 2014-2020 program Action 1.1.5.a3 with the “SENSOGM project”; EC Horizon 2020, ERA-NET - PhotonicSensing Transnational Call 2016, with the project PLABAN “advanced PLAsmonic Biosensors ANalysis of nucleic acids”; and the call for relevant instrumentation 2018 University of Florence for funding the Biacore X100 acquisition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection featuring Female Role Models in Analytical Chemistry.
Rights and permissions
About this article
Cite this article
Baldoneschi, V., Palladino, P., Scarano, S. et al. Polynorepinephrine: state-of-the-art and perspective applications in biosensing and molecular recognition. Anal Bioanal Chem 412, 5945–5954 (2020). https://doi.org/10.1007/s00216-020-02578-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02578-9