Abstract
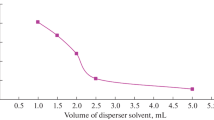
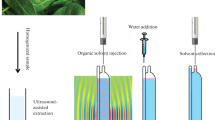
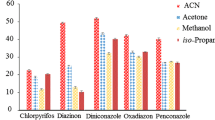
An analytical method based on ultrasound-assisted extraction and dispersive liquid–liquid microextraction (DLLME) clean-up has been developed and validated for the determination of 31 emerging pollutants in root vegetables. The target compounds were four preservatives, six perfluoroalkyl compounds, six UV filters, two biocides, eight anionic surfactants, three nonionic surfactants, and two plasticizers. The type and volume of the extraction solvent, those of the disperser solvent, the pH and NaCl content of the DLLME aqueous phase, the amount of sample, and the sonication time were optimized. Box–Behnken experimental design was applied to select the best extraction conditions. Matrix-matched calibration curves were used for quantification. Four internal standards were used to compensate for residual matrix effects. Good linearity (R2 > 0.990), accuracies (expressed as the relative recovery) of >82%, and precisions (expressed as the relative standard deviation) of <18% were achieved. Method quantification limits (MQLs), calculated from spiked samples as the concentrations corresponding to signal-to-noise ratios of 10, were in the range 0.1–25 ng g−1 dry weight (d.w.). MQL values for 26 of the 31 target compounds were lower than 5 ng g−1 d.w. The method was successfully applied to determine the target pollutants in carrots, potatoes, and turnips from a local market. To the best of our knowledge, the proposed method constitutes the first application of DLLME as a clean-up procedure for the multiresidue determination of emerging pollutants in vegetables. The method affords similar recoveries and method detection limits to previously reported methods but requires smaller solvent volumes and sample amounts and is less expensive.



Similar content being viewed by others
References
Picó Y, Alfarham A, Barceló D. Analysis of emerging contaminants and nanomaterials in plant materials following uptake from soils. Trends Anal Chem. 2017;94:173–89.
Matamoros V, Calderón-Preciado D, Domínguez C, Bayona JM. Analytical procedures for the determination of emerging organic contaminants in plant material: a review. Anal Chim Acta. 2012;722:8–20.
Prosser RS, Sibley PK. Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments and wastewater irrigation. Environ Int. 2015;75:223–33.
Wu X, Dodgen LK, Conkle JL, Gan J. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: a review. Sci Total Environ. 2015;536:655–66.
Masiá A, Suarez-Varela MM, Llopis-Gonzalez A, Picó Y. Determination of pesticides and veterinary drug residues in food by liquid chromatography–mass spectrometry: a review. Anal Chim Acta. 2016;936:40–61.
Sabourin L, Duenk P, Bonte-Gelok S, Payne M, Lapen DR, Topp E. Uptake of pharmaceuticals, hormones and parabens into vegetables grown in soil fertilized with municipal biosolids. Sci Total Environ. 2012;431:233–6.
Hurtado C, Domínguez C, Pérez-Babace L, Cañameras N, Comas J, Bayona JM. Estimate of uptake and translocation of emerging organic contaminants from irrigation water concentration in lettuce grown under controlled conditions. J Hazard Mat. 2016;305:139–48.
Jiang ZJ, Cao XL, Li H, Zhang C, El-Aty AMA, Jin F, et al. Fast determination of alkylphenol ethoxylates in leafy vegetables using a modified quick, easy, cheap, effective, rugged, and safe method and ultra-high performance supercritical fluid chromatography–tandem mass spectrometry. J Chromatogr A. 2017;1525:161–72.
Hu F, Bian K, Liu Y, Su Y, Zhou T, Song X, et al. Development of a modified quick, easy, cheap, effective, rugged and safe method for the determination of multi-class antimicrobials in vegetables by liquid chromatography tandem mass spectrometry. J Chromatogr A. 2014;1368:52–63.
Chuang YH, Zhang Y, Zhang W, Boyd SA, Li H. Comparison of accelerated solvent extraction and quick, easy, cheap, effective, rugged and safe method for extraction and determination of pharmaceuticals in vegetables. J Chromatogr A. 2015;1404:1–9.
Mijangos L, Bizkarguenaga E, Prieto A, Fernández LA, Zuloaga O. Simultaneous determination of a variety of endocrine disrupting compounds in carrot, lettuce and amended soil by means of focused ultrasonic solid–liquid extraction and dispersive solid-phase extraction as simplified clean-up strategy. J Chromatogr A. 2015;1389:8–18.
Bizkarguenaga E, Iparragirre A, Zabaleta I, Vallejo A, Fernández LA, Prieto A, et al. Focused ultrasound assisted extraction for the determination of PBDEs in vegetables and amended soil. Talanta. 2014;119:53–9.
Zabaleta I, Bizkarguenaga E, Iparragirre A, Navarro P, Prieto A, Fernández LA, et al. Focused ultrasound solid–liquid extraction for the determination of perfluorinated compounds in fish, vegetables and amended soil. J Chromatogr A. 2014;1331:27–37.
Wu X, Conkle JL, Gan J. Multi-residue determination of pharmaceutical and personal care products in vegetables. J Chromatogr A. 2012;1254:78–86.
Aparicio I, Martín J, Abril C, Santos JL, Alonso E. Determination of household and industrial chemicals, personal care products and hormones in leafy and root vegetables by liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2018;1533:49–56.
Lu J, Wu J, Stoffella PJ, Wilson PC. Isotope dilution–gas chromatography/mass spectrometry method for the analysis of alkylphenols, bisphenol A, and estrogens in food crops. J Chromatogr A. 2012;1258:128–35.
Qian ZY, Li ZG, Ma J, Gong TT, Xian QM. Analysis of trace microcystins in vegetables using matrix solid-phase dispersion followed by high performance liquid chromatography triple-quadrupole mass spectrometry detection. Talanta. 2017;173:101–6.
Albero B, Sánchez-Brunete C, Miguel E, Tadeo JL. Application of matrix solid-phase dispersion followed by GC-MS/MS to the analysis of emerging contaminants in vegetables. Food Chem. 2017;217:660–7.
Iparraguirre A, Rodil R, Quintana JB, Bizkarguenaga E, Prieto A, Zuloaga O, et al. Matrix solid-phase dispersion of polybrominated diphenyl ethers and their hydroxylated and methoxylated analogues in lettuce, carrot and soil. J Chromatogr A. 2014;1360:57–65.
Rezaee M, Assadi Y, Milani Hosseini MR, Aghaee E, Ahmadi F, Berijani S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A. 2006;1116:1–9.
Yang C, Wang J, Li D. Microextraction techniques for the determination of volatile and semivolatile organic compounds from plants: a review. Anal Chim Acta. 2013;799:8–22.
Campillo N, Viñas P, Férez-Melgarejo G, Hernández-Córdoba M. Dispersive liquid–liquid microextraction for the determination of three cytokinin compounds in fruits and vegetables by liquid chromatography with time-of-flight mass spectrometry. Talanta. 2013;116:376–81.
Pastor-Belda M, Garrido I, Campillo N, Viñas P, Hellín P, Flores P, et al. Determination of spirocyclic tetronic/tetramic acid derivatives and neonicotinoid insecticides in fruits and vegetables by liquid chromatography and mass spectrometry after dispersive liquid–liquid microextraction. Food Chem. 2016;202:389–95.
Pastor-Belda M, Garrido I, Campillo N, Viñas P, Hellín P, Flores P, et al. Combination of solvent extractants for dispersive liquid–liquid microextraction of fungicides from water and fruit samples by liquid chromatography with tandem mass spectrometry. Food Chem. 2017;233:69–76.
Ho YM, Tsoi YK, Leung KSY. Highly sensitive and selective organophosphate screening in twelve commodities of fruits, vegetables and herbal medicines by dispersive liquid–liquid microextraction. Anal Chim Acta. 2013;775:58–66.
Majlesi M, Massoudinejad M, Hosainzadeha F, Fattahi N. Simultaneous separation and preconcentration of phosalone and chlorpyrifos in fresh vegetables using ultrasound-assisted dispersive liquid–liquid microextraction and high performance liquid chromatography. Anal Methods. 2016;8:3795–801.
Pirsaheb M, Fattahi N, Shamsipur M. Determination of organophosphorous pesticides in summer crops using ultrasound-assisted solvent extraction followed by dispersive liquid–liquid microextraction based on the solidification of floating organic drop. Food Control. 2013;34:378–85.
Wu Q, Li Z, Wang C, Wu C, Wang W, Wang Z. Dispersive solid-phase extraction clean-up combined with dispersive liquid–liquid microextraction for the determination of neonicotinoid insecticides in vegetable samples by high-performance liquid chromatography. Food Anal Methods. 2011;4:559–66.
Rai S, Singh AK, Srivastava A, Yadav S, Siddiqui MH, Mudiam MKR. Comparative evaluation of QuEChERS method coupled to DLLME extraction for the analysis of multiresidue pesticides in vegetables and fruits by gas chromatography–mass spectrometry. Food Anal Methods. 2016;9:2656–69.
Viñas P, Bravo-Bravo M, López-García I, Pastor-Belda M, Hernández-Córdoba M. Pressurized liquid extraction and dispersive liquid–liquid microextraction for determination of tocopherols and tocotrienols in plant foods by liquid chromatography with fluorescence and atmospheric pressure chemical ionization–mass spectrometry detection. Talanta. 2014;119:98–104.
Tijani JO, Fatoba OO, Babajide OO, Petrik LF. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: a review. Environ Chem Lett. 2016;14:27–49.
Martín J, Zafra-Gómez A, Hidalgo F, Ibáñez-Yuste AJ, Alonso E, Vilchez JL. Multi-residue analysis of 36 priority and emerging pollutants in marine echinoderms (Holothuria tubulosa) and marine sediments by solid–liquid extraction followed by dispersive solid phase extraction and liquid chromatography–tandem mass spectrometry analysis. Talanta. 2017;166:336–48.
Stalikas C, Fiamegos Y, Sakkas V, Albanis T. Developments on chemometric approaches to optimize and evaluate microextraction. J Chromatogr A. 2009;1216:175–89.
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão JC, et al. Box–Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta. 2007;597:179–86.
García-Galán MJ, Petrovic M, Rodríguez-Mozaz S, Barceló D. Multiresidue trace analysis of pharmaceuticals, their human metabolites and transformation products by fully automated on-line solid-phase extraction–liquid chromatography–tandem mass spectrometry. Talanta. 2016;158:330–41.
Prosser RS, Lissemore L, Topp E, Sibley PK. Bioaccumulation of triclosan and triclocarban in plants grown in soils amended with municipal dewatered biosolids. Environ Toxicol Chem. 2014;33:975–84.
Acknowledgments
This work was financially supported by the Spanish Ministry of Economy and Competitiveness (project no. CTM2017-82778-R).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PDF 1461 kb)
Rights and permissions
About this article
Cite this article
Abril, C., Martín, J., Malvar, J.L. et al. Dispersive liquid–liquid microextraction as a new clean-up procedure for the determination of parabens, perfluorinated compounds, UV filters, biocides, surfactants, and plasticizers in root vegetables. Anal Bioanal Chem 410, 5155–5163 (2018). https://doi.org/10.1007/s00216-018-1165-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1165-9