Abstract
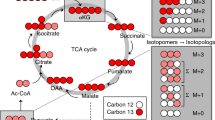
Isotope labeling enables the use of 13C-based metabolomics techniques with strongly improved resolution for a better identification of relevant metabolites and tracing of metabolic fluxes in cell and animal models, as required in fluxomics studies. However, even at high NMR-active isotope abundance, the acquisition of one-dimensional 13C and classical two-dimensional 1H,13C-HSQC experiments remains time consuming. With the aim to provide a shorter, more efficient alternative, herein we explored the ALSOFAST-HSQC experiment with its rapid acquisition scheme for the analysis of 13C-labeled metabolites in complex biological mixtures. As an initial step, the parameters of the pulse sequence were optimized to take into account the specific characteristics of the complex samples. We then applied the fast two-dimensional experiment to study the effect of different kinds of antioxidant gold nanoparticles on a HeLa cancer cell model grown on 13C glucose-enriched medium. As a result, 1H,13C-2D correlations could be obtained in a couple of seconds to few minutes, allowing a simple and reliable identification of various 13C-enriched metabolites and the determination of specific variations between the different sample groups. Thus, it was possible to monitor glucose metabolism in the cell model and study the antioxidant effect of the coated gold nanoparticles in detail. Finally, with an experiment time of only half an hour, highly resolved 1H,13C-HSQC spectra using the ALSOFAST-HSQC pulse sequence were acquired, revealing the isotope-position-patterns of the corresponding 13C-nuclei from carbon multiplets.

Fast NMR applied to metabolomics and fluxomics studies with gold nanoparticles







Similar content being viewed by others
References
Kaddurah-Daouk R, Krishnan KR. Metabolomics: a global biochemical approach to the study of central nervous system diseases. Neuropsychopharmacology. 2009;34(1):173–86.
Wishart DS. Applications of metabolomics in drug discovery and development. Drugs R D. 2008;9(5):307–22.
Hall RD, Brouwer ID, Fitzgerald MA. Plant metabolomics and its potential application for human nutrition. Physiol Plant. 2008;132(2):162–75.
Cevallos-Cevallos JM, Rouseff R, Reyes-De-Corcuera JI. Untargeted metabolite analysis of healthy and Huanglongbing-infected orange leaves by CE-DAD. Electrophoresis. 2009;30(7):1240–7.
Rivas-Ubach A, Perez-Trujillo M, Sardans J, Gargallo-Garriga A, Parella T, Penuelas J. Ecometabolomics: optimized NMR-based method. Methods Ecol Evol. 2013;4(5):464–73.
Bollard ME, Stanley EG, Lindon JC, Nicholson JK, Holmes E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed. 2005;18(3):143–62.
Yuan J, Bennett BD, Rabinowitz JD. Kinetic flux profiling for quantitation of cellular metabolic fluxes. Nat Protoc. 2008;3(8):1328–40.
Zwingmann C, Leibfritz D. Regulation of glial metabolism studied by 13C-NMR. NMR Biomed. 2003;16(6–7):370–99.
Jans AW, Leibfritz D. A 13C NMR study on fluxes into the Krebs cycle of rabbit renal proximal tubular cells. NMR Biomed. 1989;1(4):171–6.
Heuner K, Eisenreich W. The intracellular metabolism of legionella by isotopologue profiling. Methods Mol Biol. 2013;954:163–81.
Niedenfuhr S, Wiechert W, Noh K. How to measure metabolic fluxes: a taxonomic guide for (13)C fluxomics. Curr Opin Biotechnol. 2015;34:82–90.
Mahrous EA, Farag MA. Two dimensional NMR spectroscopic approaches for exploring plant metabolome: a review. J Adv Res. 2015;6(1):3–15.
Gómez J, Brezmes J, Mallol R, Rodríguez MA, Vinaixa M, Salek RM, et al. Dolphin: a tool for automatic targeted metabolite profiling using 1D and 2D (1)H-NMR data. Anal Bioanal Chem. 2014;406(30):7967–76.
Bingol K, Li D, Zhang B, Bruschweiler R. Comprehensive metabolite identification strategy using multiple two-dimensional NMR spectra of a complex mixture implemented in the COLMARm web server. Anal Chem. 2016;88(24):12411–8.
Massou S, Nicolas C, Letisse F, Portais JC. NMR-based fluxomics: quantitative 2D NMR methods for isotopomers analysis. Phytochemistry. 2007;68(16–18):2330–40.
Nath J, Smith T, Hollis A, Ebbs S, Canbilen SW, Tennant DA, et al. Ludwig C: (13)C glucose labelling studies using 2D NMR are a useful tool for determining ex vivo whole organ metabolism during hypothermic machine perfusion of kidneys. Transplant Res. 2016;5:7.
Giraudeau P, Frydman L. Ultrafast 2D NMR: an emerging tool in analytical spectroscopy. Annu Rev Anal Chem (Palo Alto, Calif). 2014;7:129–61.
Frueh DP, Goodrich AC, Mishra SH, Nichols SR. NMR methods for structural studies of large monomeric and multimeric proteins. Curr Opin Struct Biol. 2013;23(5):734–9.
Vitorge B, Bieri S, Humam M, Christen P, Hostettmann K, Munoz O, et al. High-precision heteronuclear 2D NMR experiments using 10-ppm spectral window to resolve carbon overlap. Chem Commun (Camb). 2009;8:950–2.
Schanda P. Fast-pulsing longitudinal relaxation optimized techniques: enriching the toolbox of fast biomolecular NMR spectroscopy. Prog Nucl Mag Res Spec. 2009;55:238–65.
Vitorge B, Bodenhausen G, Pelupessy P. Speeding up nuclear magnetic resonance spectroscopy by the use of SMAll recovery times—SMART NMR. J Magn Reson. 2010;207(1):149–52.
Mueller L. Alternate HMQC experiments for recording HN and HC-correlation spectra in proteins at high throughput. J Biomol NMR. 2008;42(2):129–37.
Schulze-Sünninghausen D, Becker J, Koos MRM, Luy B. Improvements, extensions, and practical aspects of rapid ASAP-HSQC and ALSOFAST-HSQC pulse sequences for studying small molecules at natural abundance. J Magn Reson. 2017;281:151–61.
Schulze-Sünnighausen D, Becker J, Luy B. Rapid heteronuclear single quantum correlation NMR at natural abundance. J Am Chem Soc. 2014;136:1242–5.
Kupce E, Freeman R. SPEED: single point evaluation of the evolution dimension. Mag Reson Chem. 2007;45:711–3.
Becker J, Luy B. CLIP-ASAP-HSQC for fast and accurate extraction of one-bond couplings from isotropic and partially aligned molecules. Magn Reson Chem. 2015;53(11):878–85.
Marchand J, Martineau E, Guitton Y, Dervilly-Pinel G, Giraudeau P. Multidimensional NMR approaches towards highly resolved, sensitive and high-throughput quantitative metabolomics. Curr Opin Biotechnol. 2017;43:49–55.
Le Guennec A, Dumez JN, Giraudeau P, Caldarelli S. Resolution-enhanced 2D NMR of complex mixtures by non-uniform sampling. Magn Reson Chem. 2015;53(11):913–20.
Le Guennec A, Tea I, Antheaume I, Martineau E, Charrier B, Pathan M, et al. Fast determination of absolute metabolite concentrations by spatially encoded 2D NMR: application to breast cancer cell extracts. Anal Chem. 2012;84(24):10831–7.
Rai RK, Sinha N. Fast and accurate quantitative metabolic profiling of body fluids by nonlinear sampling of 1H-13C two-dimensional nuclear magnetic resonance spectroscopy. Anal Chem. 2012;84(22):10005–11.
Sharma R, Gogna N, Singh H, Dorai K. Fast profiling of metabolite mixtures using chemometric analysis of a speeded-up 2D heteronuclear correlation NMR experiment. RSC Adv. 2017;7:29860–70.
Zhang A, Sun H, Xu H, Qiu S, Wang X. Cell metabolomics. OMICS. 2013;17(10):495–501.
Murday JS, Siegel RW, Stein J, Wright JF. Translational nanomedicine: status assessment and opportunities. Nanomedicine. 2009;5(3):251–73.
Fadeel B, Garcia-Bennett AE. Better safe than sorry: understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv Drug Deliv Rev. 2010;62(3):362–74.
Menchón C, Martín R, Apostolova N, Victor VM, Alvaro M, Herance JR, et al. Gold nanoparticles supported on nanoparticulate ceria as a powerful agent against intracellular oxidative stress. Small. 2012;8(12):1895–903.
Esumi K, Takei N, Yoshimura T. Antioxidant-potentiality of gold-chitosan nanocomposites. Colloids and Surfaces B-Biointerfaces. 2003;32(2):117–23.
Atreya HS, Szyperski T. G-matrix Fourier transform NMR spectroscopy for complete protein resonance assignment. Proc Natl Acad Sci U S A. 2004;101(26):9642–7.
Marion D. Fast acquisition of NMR spectra using Fourier transform of non-equispaced data. J Biomol NMR. 2005;32(2):141–50.
Kazimierczuk K, Orekhov VY. Accelerated NMR spectroscopy by using compressed sensing. Angew Chem Int Ed Engl. 2011;50(24):5556–9.
SHAKA A, BARKER P, FREEMAN R. Computer-optimized decoupling scheme for wideband applications and low-level operation. J Magn Reson. 1985;64(3):547–52.
Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, et al. HMDB 3.0—the human metabolome database in 2013. Nucleic Acids Res. 2013;41(Database issue):D801–7.
Ulrich EL, Akutsu H, Doreleijers JF, Harano Y, Ioannidis YE, Lin J, et al. BioMagResBank. Nucleic Acids Res. 2008;36(Database issue):D402–8.
Liu J, Segal MR, Kelly MJ, Pelton JG, Kim M, James TL, et al. 13C NMR metabolomic evaluation of immediate and delayed mild hypothermia in cerebrocortical slices after oxygen-glucose deprivation. Anesthesiology. 2013;119(5):1120–36.
Faiz H, Conjard-Duplany A, Boghossian M, Martin G, Baverel G, Ferrier B. Cadmium chloride inhibits lactate gluconeogenesis in isolated human renal proximal tubules: a cellular metabolomic approach with 13C-NMR. Arch Toxicol. 2011;85(9):1067–77.
Marion D, Wüthrich K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem Biophys Res Commun. 1983;113(3):967–74.
Ernst RR, Bodenhausen G, Wokaun A: Principles of nuclear magnetic resonance in one and two dimensions, vol. 14; 1987.
Pilatus U, Aboagye E, Artemov D, Mori N, Ackerstaff E, Bhujwalla ZM. Real-time measurements of cellular oxygen consumption, pH, and energy metabolism using nuclear magnetic resonance spectroscopy. Magn Reson Med. 2001;45(5):749–55.
Lucas LH, Larive CK, Wilkinson PS, Huhn S. Progress toward automated metabolic profiling of human serum: comparison of CPMG and gradient-filtered NMR analytical methods. J Pharm Biomed Anal. 2005;39(1–2):156–63.
Barathmanikanth S, Kalishwaralal K, Sriram M, Pandian SR, Youn HS, Eom S, et al. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J Nanobiotechnology. 2010;8:16.
Boccard J, Rutledge DN. A consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal Chim Acta. 2013;769:30–9.
Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, Edlund U, Shockcor JP, et al. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem. 2008;80(1):115–22.
Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the "O-GlcNAc code". Sci STKE. 2005;2005(312):re13.
Wells L, Vosseller K, Hart GW. A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol Life Sci. 2003;60(2):222–8.
Acknowledgements
Part of the work was supported by grants CP13/00252 and PI16/02064 from Carlos III Health Institute, SAF2014-53977-R and SAF2017-89229-R from Ministerio de Economía y Competitividad, and by the European Regional Development Fund (ERDF). M.P.-S. was partially supported by an EMBO short-term fellowship. Eva Castelló has contributed to the work with technical help. B.L. thanks the HGF programme BIFTM, the DFG (instrumentation facility Pro2NMR and LU 835/13-1), and Fonds der Chemischen Industrie for financial support. We also want to thank Martin Koos for helpful discussions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 645 kb)
Rights and permissions
About this article
Cite this article
Schätzlein, M.P., Becker, J., Schulze-Sünninghausen, D. et al. Rapid two-dimensional ALSOFAST-HSQC experiment for metabolomics and fluxomics studies: application to a 13C-enriched cancer cell model treated with gold nanoparticles. Anal Bioanal Chem 410, 2793–2804 (2018). https://doi.org/10.1007/s00216-018-0961-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0961-6