Abstract
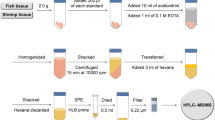
This work describes the optimization, validation, and application in real samples of accurate and precise analytical methods to determine ten fluoroquinolones (FQs) (norfloxacin, enoxacin, pefloxacin, ofloxacin, levofloxacin, ciprofloxacin, danofloxacin, lomefloxacin, enrofloxacin, and sparfloxacin) in different environmental matrices, such as water (estuarine, seawater, and wastewater treatment plant effluent), fish tissues (muscle and liver), and fish biofluids (plasma and bile). The analysis step performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) was fully optimized to improve the separation and detection steps. The extraction of analytes from fish tissues was accomplished using focused ultrasound solid-liquid extraction using methanol/acetic acid (95:5 v/v) as extractant. The preconcentration and clean-up steps were optimized in terms of extraction efficiency and cleanliness and the best strategy for each matrix was selected: (i) Oasis HLB for seawater and muscle, (ii) liquid-liquid extraction combined with Oasis HLB for the lipid-rich liver, (iii) the combination of Evolute-WAX and Oasis HLB for estuarine water and wastewater treatment plant effluent, and (iv) molecular imprinted polymers for biofluids. The methods afforded satisfactory apparent recoveries (80–126%) and repeatability (RSD < 15%), except for sparfloxacin, which showed a lack of correction with the available isotopically labeled surrogates ([2H8]–ciprofloxacin and [2H5]–enrofloxacin). Ciprofloxacin, norfloxacin, and ofloxacin were detected in both water and fish liver samples from the Biscay Coast at concentrations up to 278 ng/L and 4 ng/g, respectively. To the best of our knowledge, this work is one of the few analyzing up to ten FQs and in so many fish tissues and biofluids.

Determination of fluoroquinolones in different environmental matrices, such as water (estuarine, seawater, and wastewater treatment plant effluent), fish tissues (muscle and liver), and fish biofluids (plasma and bile).




Similar content being viewed by others
References
Vazquez-Roig P, Blasco C, Picó Y. Advances in the analysis of legal and illegal drugs in the aquatic environment. TrAC Trends Anal Chem. 2013;50:65–77.
Besse J-P, Garric J. Human pharmaceuticals in surface waters: implementation of a prioritization methodology and application to the French situation. Toxicol Lett. 2008;176:104–23.
Seifrtová M, Nováková L, Lino C, Pena A, Solich P. An overview of analytical methodologies for the determination of antibiotics in environmental waters. Anal Chim Acta. 2009;649:158–79.
Van Doorslaer X, Dewulf J, Van Langenhove H, Demeestere K. Fluoroquinolone antibiotics: an emerging class of environmental micropollutants. Sci Total Environ. 2014;500–501:250–69.
Martinez M, McDermott P, Walker R. Pharmacology of the fluoroquinolones: a perspective for the use in domestic animals. Vet J. 2006;172:10–28.
Speltini A, Sturini M, Maraschi F, Profumo A. Fluoroquinolone antibiotics in environmental waters: sample preparation and determination. J Sep Sci. 2010;33:1115–31.
Andreozzi R, Raffaele M, Nicklas P. Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere. 2003;50:1319–30.
Petrović M, Hernando MD, Díaz-Cruz MS, Barceló D. Liquid chromatography–tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: a review. J Chromatogr A. 2005;1067:1–14.
Turiel E, Martín-Esteban A, Tadeo JL. Multiresidue analysis of quinolones and fluoroquinolones in soil by ultrasonic-assisted extraction in small columns and HPLC-UV. Anal Chim Acta. 2006;562:30–5.
Janecko N, Pokludova L, Blahova J, Svobodova Z, Literak I. Implications of fluoroquinolone contamination for the aquatic environment—a review. Environ Toxicol Chem. 2016;35:2647–56.
Sturini M, Speltini A, Pretali L, Fasani E, Profumo A. Solid-phase extraction and HPLC determination of fluoroquinolones in surface waters. J Sep Sci. 2009;32:3020–8.
Benito-Peña E, Urraca JL, Sellergren B, Moreno-Bondi MC. Solid-phase extraction of fluoroquinolones from aqueous samples using a water-compatible stochiometrically imprinted polymer. J Chromatogr A. 2008;1208:62–70.
Lee H-B, Peart TE, Svoboda ML. Determination of ofloxacin, norfloxacin, and ciprofloxacin in sewage by selective solid-phase extraction, liquid chromatography with fluorescence detection, and liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2007;1139:45–52.
Nakata H, Kannan K, Jones PD, Giesy JP. Determination of fluoroquinolone antibiotics in wastewater effluents by liquid chromatography–mass spectrometry and fluorescence detection. Chemosphere. 2005;58:759–66.
Wagil M, Kumirska J, Stolte S, Puckowski A, Maszkowska J, Stepnowski P, et al. Development of sensitive and reliable LC-MS/MS methods for the determination of three fluoroquinolones in water and fish tissue samples and preliminary environmental risk assessment of their presence in two rivers in northern Poland. Sci Total Environ. 2014;493:1006–13.
Zhao J-L, Liu Y-S, Liu W-R, Jiang Y-X, Su H-C, Zhang Q-Q, et al. Tissue-specific bioaccumulation of human and veterinary antibiotics in bile, plasma, liver and muscle tissues of wild fish from a highly urbanized region. Environ Pollut. 2015;198:15–24.
EMEA. Committee for veterinary medicinal products, Enrofloxacin EMEA/MRL/820/02-FINAL. http://www.ema.europa.eu/ema/index.jsp?curl=pages/includes/document/document_detail.jsp?webContentId=WC500014151&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058009a3dc (2002). Cited 28 Feb 2017.
Huerta B, Rodríguez-Mozaz S, Barceló D. Pharmaceuticals in biota in the aquatic environment: analytical methods and environmental implications. Anal Bioanal Chem. 2012;404:2611–24.
Yu H, Tao Y, Chen D, Pan Y, Liu Z, Wang Y, et al. Simultaneous determination of fluoroquinolones in foods of animal origin by a high performance liquid chromatography and a liquid chromatography tandem mass spectrometry with accelerated solvent extraction. J Chromatogr B. 2012;885–886:150–9.
Boscher A, Guignard C, Pellet T, Hoffmann L, Bohn T. Development of a multi-class method for the quantification of veterinary drug residues in feedingstuffs by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2010;1217:6394–404.
Speltini A, Sturini M, Maraschi F, Profumo A, Albini A. Analytical methods for the determination of fluoroquinolones in solid environmental matrices. TrAC Trends Anal Chem. 2011;30:1337–50.
Cho H-J, Yi H, Cho SM, Lee DG, Cho K, Abd El-Aty AM, et al. Single-step extraction followed by LC for determination of (fluoro)quinolone drug residues in muscle, eggs, and milk. J Sep Sci. 2010;33:1034–43.
Evaggelopoulou EN, Samanidou VF. HPLC confirmatory method development for the determination of seven quinolones in salmon tissue (Salmo Salar L.) validated according to the European Union Decision 2002/657/EC. Food Chem. 2013;136:479–84.
Sun X, Wang J, Li Y, Yang J, Jin J, Shah SM, et al. Novel dummy molecularly imprinted polymers for matrix solid-phase dispersion extraction of eight fluoroquinolones from fish samples. J Chromatogr A. 2014;1359:1–7.
Zabaleta I, Bizkarguenaga E, Iparragirre A, Navarro P, Prieto A, Fernández LÁ, et al. Focused ultrasound solid–liquid extraction for the determination of perfluorinated compounds in fish, vegetables and amended soil. J Chromatogr A. 2014;1331:27–37.
Ziarrusta H, Mijangos L, Prieto A, Etxebarria N, Zuloaga O, Olivares M. Determination of tricyclic antidepressants in biota tissue and environmental waters by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2015;408:1205–16.
Chiesa L, Nobile M, Arioli F, Britti D, Trutic N, Pavlovic R, et al. Determination of veterinary antibiotics in bovine urine by liquid chromatography–tandem mass spectrometry. Food Chem. 2015;185:7–15.
Bourdat-Deschamps M, Leang S, Bernet N, Daudin J-J, Nélieu S. Multi-residue analysis of pharmaceuticals in aqueous environmental samples by online solid-phase extraction–ultra-high-performance liquid chromatography-tandem mass spectrometry: optimisation and matrix effects reduction by quick, easy, cheap, effective, rugged and safe extraction. J Chromatogr A. 2014;1349:11–23.
Tamtam F, Mercier F, Eurin J, Chevreuil M, Bot BL. Ultra performance liquid chromatography tandem mass spectrometry performance evaluation for analysis of antibiotics in natural waters. Anal Bioanal Chem. 2009;393:1709–18.
Calisto V, Esteves VI. Psychiatric pharmaceuticals in the environment. Chemosphere. 2009;77:1257–74.
Rodríguez E, Navarro-Villoslada F, Benito-Peña E, Marazuela MD, Moreno-Bondi MC. Multiresidue determination of ultratrace levels of fluoroquinolone antimicrobials in drinking and aquaculture water samples by automated online molecularly imprinted solid phase extraction and liquid chromatography. Anal Chem. 2011;83:2046–55.
Speltini A, Sturini M, Maraschi F, Consoli L, Zeffiro A, Profumo A. Graphene-derivatized silica as an efficient solid-phase extraction sorbent for pre-concentration of fluoroquinolones from water followed by liquid-chromatography fluorescence detection. J Chromatogr A. 2015;1379:9–15.
Prieto A, Schrader S, Bauer C, Möder M. Synthesis of a molecularly imprinted polymer and its application for microextraction by packed sorbent for the determination of fluoroquinolone related compounds in water. Anal Chim Acta. 2011;685:146–52.
Application note for Biotage-AFFINILUTE™ MIP Fluoroquinolones. http://www.biotage.com/product-page/affinilute-mip-columns. Cited 28 Feb 2017.
Gros M, Rodríguez-Mozaz S, Barceló D. Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J Chromatogr A. 2013;1292:173–88.
Golet EM, Strehler A, Alder AC, Giger W. Determination of fluoroquinolone antibacterial agents in sewage sludge and sludge-treated soil using accelerated solvent extraction followed by solid-phase extraction. Anal Chem. 2002;74:5455–62.
Tang Q, Yang T, Tan X, Luo J. Simultaneous determination of fluoroquinolone antibiotic residues in milk sample by solid-phase extraction−liquid chromatography−tandem mass spectrometry. J Agric Food Chem. 2009;57:4535–9.
He X, Wang Z, Nie X, Yang Y, Pan D, Leung AOW, et al. Residues of fluoroquinolones in marine aquaculture environment of the Pearl River Delta. South China Environ Geochem Health. 2011;34:323–35.
Acknowledgements
This work was financially supported by the Ministry of Economy and Competitiveness through the project CTM2014-56628-C3-1-R and by the Basque Government through the project IT-742-13. H. Ziarrusta is grateful to the Spanish Ministry and L. Mijangos to the Basque Government for their pre-doctoral fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ziarrusta, H., Val, N., Dominguez, H. et al. Determination of fluoroquinolones in fish tissues, biological fluids, and environmental waters by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 409, 6359–6370 (2017). https://doi.org/10.1007/s00216-017-0575-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0575-4