Abstract
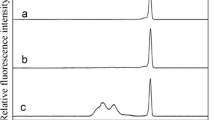
In this work, we characterized different phtalocyanine-capped core/shell/shell quantum dots (QDs) in terms of stability, ζ-potential, and size at various pH and ionic strengths, by means of capillary electrophoresis (CE), and compared these results to the ones obtained by laser Doppler electrophoresis (LDE) and dynamic light scattering (DLS). The effect of the phthalocyanine metallic center (Zn, Al, or In), the number (one or four), and nature of substituents (carboxyphenoxy- or sulfonated-) of functionalization on the phthalocyanine physicochemical properties were evaluated. Whereas QDs capped with zinc mono-carboxyphenoxy-phtalocyanine (ZnMCPPc-QDs) remained aggregated in the whole analyzed pH range, even at low ionic strength, QDs capped with zinc tetracarboxyphenoxy phtalocyanine (ZnTPPc-QDs) were easily dispersed in buffers at pH equal to or higher than 7.4. QDs capped with aluminum tetrasulfonated phthalocyanine (AlTSPPc-QDs) and indium tetracarboxyphenoxy phthalocyanines (InTCPPc-QDs) were stable in aqueous suspension only at pH higher than 9.0 due to the presence of functional groups bound to the metallic center of the phthalocyanine. The ζ-potential values determined by CE for all the samples decreased when ionic strength increased, being well correlated with the aggregation of the nanoconjugates at elevated salt concentrations. The use of electrokinetic methodologies has provided insights into the colloidal stability of the photosensitizer-functionalized QDs in physiological relevant solutions and thereby, its usefulness for improving their design and applications for photodynamic therapy.

Schematic illustration of the phthalocyanine capped QDs nanoconjugates and the capillary electrophoresis methods applied for size and ζ-potential characterization





Similar content being viewed by others
References
Breger J, Delehanty JB, Medintz IL. Continuing progress toward controlled intracellular delivery of semiconductor quantum dots. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(2):131–51. doi:10.1002/wnan.1281.
Mussa Farkhani S, Valizadeh A. Review: three synthesis methods of CdX (X = Se, S or Te) quantum dots. IET Nanobiotechnol. 2014;8(2):59–76. doi:10.1049/iet-nbt.2012.0028.
Volkov Y. Quantum dots in nanomedicine: recent trends, advances and unresolved issues. Biochem Biophys Res Commun. 2015;468(3):419–27. doi:10.1016/j.bbrc.2015.07.039.
Maysinger D, Ji J, Hutter E, Cooper E. Nanoparticle-based and bioengineered probes and sensors to detect physiological and pathological biomarkers in neural cells. Front Neurosci. 2015;9:480. doi:10.3389/fnins.2015.00480.
Petryayeva E, Algar WR, Medintz IL. Quantum dots in bioanalysis: a review of applications across various platforms for fluorescence spectroscopy and imaging. Appl Spectrosc. 2013;67(3):215–52. doi:10.1366/12-06948.
Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev. 2015;115(4):1990–2042. doi:10.1021/cr5004198.
Oluwole DO, Nyokong T. Physicochemical behavior of nanohybrids of mono and tetra substituted carboxyphenoxy phthalocyanine covalently linked to GSH–CdTe/CdS/ZnS quantum dots. Polyhedron. 2015;87:8–16. doi:10.1016/j.poly.2014.10.024.
Oluwole DO, Nyokong T. Comparative photophysicochemical behavior of nanoconjugates of indium tetracarboxyphenoxy phthalocyanines covalently linked to CdTe/ZnSe/ZnO quantum dots. J Photoch Photobio A. 2015;312:34–44. doi:10.1016/j.jphotochem.2015.07.009.
Oluwole DO, Britton J, Mashazi P, Nyokong T. Synthesis and photophysical properties of nanocomposites of aluminum tetrasulfonated phthalocyanine covalently linked to glutathione capped CdTe/CdS/ZnS quantum dots. Synth Met. 2015;205:212–21. doi:10.1016/j.synthmet.2015.04.015.
Li L, Huh KM. Polymeric nanocarrier systems for photodynamic therapy. Biomater Res. 2014;18.
Lopez-Serrano A, Olivas RM, Landaluze JS, Camara C. Nanoparticles: a global vision. Characterization, separation, and quantification methods. Potential environmental and health impact. Anal Methods. 2014;6(1):38–56. doi:10.1039/C3AY40517F.
Ban E, Yoo YS, Song EJ. Analysis and applications of nanoparticles in capillary electrophoresis. Talanta. 2015;141:15–20. doi:10.1016/j.talanta.2015.03.020.
Trapiella-Alfonso L, d’Orlyé F, Varenne A. Recent advances in the development of capillary electrophoresis methodologies for optimizing, controlling, and characterizing the synthesis, functionalization, and physicochemical, properties of nanoparticles. Anal Bioanal Chem. 2016:1-7. doi:10.1007/s00216-015-9236-7.
Li YQ, Wang HQ, Wang JH, Guan LY, Liu BF, Zhao YD, et al. A highly efficient capillary electrophoresis-based method for size determination of water-soluble CdSe/ZnS core-shell quantum dots. Anal Chim Acta. 2009;647(2):219–25. doi:10.1016/j.aca.2009.06.004.
Stewart DTR, Celiz MD, Vicente G, Colón LA, Aga DS. Potential use of capillary zone electrophoresis in size characterization of quantum dots for environmental studies. Tr Anal Chem. 2011;30(1):113–22. doi:10.1016/j.trac.2010.10.005.
Sang F, Huang X, Ren J. Characterization and separation of semiconductor quantum dots and their conjugates by capillary electrophoresis. Electrophoresis. 2014;35(6):793–803. doi:10.1002/elps.201300528.
Radko SP, Chrambach A. Separation and characterization of sub-mu m- and mu m-sized particles by capillary zone electrophoresis. Electrophoresis. 2002;23(13):1957–72. doi:10.1002/1522-2683(200207)23:13<1957::aid-elps1957>3.0.co;2-i.
d’Orlye F, Varenne A, Georgelin T, Siaugue JM, Teste B, Descroix S, et al. Charge-based characterization of nanometric cationic bifunctional maghemite/silica core/shell particles by capillary zone electrophoresis. Electrophoresis. 2009;30(14):2572–82. doi:10.1002/elps.200800835.
d’Orlyé F, Varenne A, Gareil P. Determination of nanoparticle diffusion coefficients by Taylor dispersion analysis using a capillary electrophoresis instrument. J Chromatogr A. 2008;1204(2):226–32. doi:10.1016/j.chroma.2008.08.008.
Wang YH, Wang L. Defect states in Nd3+-doped CaAl2O4 : Eu2+. J Appl Phys. 2007;101(5). doi: 10.1063/1.2435822.
Fourest B, Hakem N, Guillaumont R. Characterization of colloids by measurement of their mobilities. Radiochim Acta. 1994. p. 173.
Huang X, Weng J, Sang F, Song X, Cao C, Ren J. Characterization of quantum dot bioconjugates by capillary electrophoresis with laser-induced fluorescent detection. J Chromatogr A. 2006;1113(1-2):251–4. doi:10.1016/j.chroma.2006.02.087.
Trapiella-Alfonso L, Ramírez-García G, d’Orlyé F, Varenne A. Electromigration separation methodologies for the characterization of nanoparticles and the evaluation of their behaviour in biological systems. Tr Anal Chem. 2016;84(Part A):121–30. doi:10.1016/j.trac.2016.04.022.
Ramirez-Garcia G, d’Orlye F, Gutierrez-Granados S, Martinez-Alfaro M, Mignet N, Richard C, et al. Functionalization and characterization of persistent luminescence nanoparticles by dynamic light scattering, laser Doppler and capillary electrophoresis. Colloid Surface B. 2015;136:272–81. doi:10.1016/j.colsurfb.2015.09.022.
Rivera Gil P, Oberdörster G, Elder A, Puntes V, Parak WJ. Correlating physico-chemical with toxicological properties of nanoparticles: the present and the future. ACS Nano. 2010;4(10):5527–31. doi:10.1021/nn1025687.
Powers KW, Brown SC, Krishna VB, Wasdo SC, Moudgil BM, Roberts SM. Research strategies for safety evaluation of nanomaterials. Part VI. Characterization of nanoscale particles for toxicological evaluation. Toxicol Sci. 2006;90(2):296–303. doi:10.1093/toxsci/kfj099.
Nam J, Won N, Bang J, Jin H, Park J, Jung S, et al. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv Drug Deliv Rev. 2013;65(5):622–48. doi:10.1016/j.addr.2012.08.015.
Sperling RA, Parak WJ. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos T Roy Soc A. 2010;368(1915):1333–83. doi:10.1098/rsta.2009.0273.
Hondow N, Brydson R, Wang P, Holton MD, Brown MR, Rees P, et al. Quantitative characterization of nanoparticle agglomeration within biological media. J Nanopart Res. 2012;14(7):1–15. doi:10.1007/s11051-012-0977-3.
Milanova D, Chambers RD, Bahga SS, Santiago JG. Electrophoretic mobility measurements of fluorescent dyes using on-chip capillary electrophoresis. Electrophoresis. 2011;32(22):3286–94. doi:10.1002/elps.201100210.
Pyell U, Jalil AH, Pfeiffer C, Pelaz B, Parak WJ. Characterization of gold nanoparticles with different hydrophilic coatings via capillary electrophoresis and Taylor dispersion analysis. Part I: determination of the zeta potential employing a modified analytic approximation. J Colloid Interface Sci. 2015;450:288–300. doi:10.1016/j.jcis.2015.03.006.
Pyell U, Jalil AH, Urban DA, Pfeiffer C, Pelaz B, Parak WJ. Characterization of hydrophilic coated gold nanoparticles via capillary electrophoresis and Taylor dispersion analysis. Part II: determination of the hydrodynamic radius distribution—comparison with asymmetric flow field-flow fractionation. J Colloid Interface Sci. 2015;457:131–40. doi:10.1016/j.jcis.2015.06.042.
Taylor G. Dispersion of soluble matter in solvent flowing slowly through a tube. P Roy Soc Lond A Mat. 1953;219(1137):186–203. doi:10.1098/rspa.1953.0139.
Aris R. On the dispersion of a solute in a fluid flowing through a tube. P Roy Soc Lond A Mat. 1956;235(1200):67–77. doi:10.1098/rspa.1956.0065.
Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63(6):456–69. doi:10.1016/j.addr.2011.02.001.
Kuzovkov VN, Kotomin EA. Static and dynamic screening effects in the electrostatic self-assembly of nano-particles. Phys Chem Chem Phys. 2014;16(46):25449–60. doi:10.1039/C4CP02448F.
Brar SK, Verma M. Measurement of nanoparticles by light-scattering techniques. TrAC T Anal Chem. 2011;30(1):4–17. doi:10.1016/j.trac.2010.08.008.
Hoo CM, Starostin N, West P, Mecartney ML. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J Nanopart Res. 2008;10(1):89–96. doi:10.1007/s11051-008-9435-7.
Sapsford KE, Tyner KM, Dair BJ, Deschamps JR, Medintz IL. Analyzing nanomaterial bioconjugates: a review of current and emerging purification and characterization techniques. Anal Chem. 2011;83(12):4453–88. doi:10.1021/ac200853a.
Acknowledgments
This work was partially supported by the Department of Science and Technology (DST) and National Research Foundation (NRF) of South Africa, through the DST/NRF South African Research Chairs Initiative for Professor of Medicinal Chemistry and Nanotechnology (UID=62620) and Rhodes University and by DST/Mintek Nanotechnology Innovation Centre (NIC). SRN acknowledges the financial contribution of the DST women in science masters’ fellowship award and the NRF towards this research. GRG is grateful to the Mexican National Council for Science and Technology (CONACYT) for doctoral fellowship. The authors acknowledge financial support from PROTEA Project 33885ZJ (France–South Africa).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ramírez-García, G., Oluwole, D.O., Nxele, S.R. et al. Characterization of phthalocyanine functionalized quantum dots by dynamic light scattering, laser Doppler, and capillary electrophoresis. Anal Bioanal Chem 409, 1707–1715 (2017). https://doi.org/10.1007/s00216-016-0120-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0120-x