Abstract
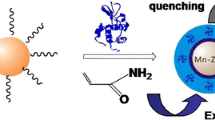
The direct correlation between disease and lysozyme (LYZ) levels in human body fluids makes the sensitive and convenient detection of LYZ the focus of scientific research. Fluorescent molecularly imprinted polymer has emerged as a new alternative for LYZ detection in order to resolve the limitation of immunoassays, which are expensive, unstable, require complex preparation, and are time consuming. In this study, a novel fluorescence molecularly imprinted polymer based on Navicula sp. frustules (FITC-MIP) has been synthesized via post-imprinting treatment for LYZ detection. Navicula sp. frustules were used as supported material because of their unique properties of moderate surface area, reproducibility, and biocompatibility, to address the drawbacks of nanoparticle core material with low adsorption capacity. The FITC acts as recognition signal and optical readout, whereas MIP provides LYZ selectivity. The synthesized FITC-MIP showed a response time as short as 5 min depending on the concentration of LYZ. It is found that the LYZ template can significantly quench the fluorescence intensity of FITC-MIP linearly within a concentration range of 0 to 0.025 mg mL–1, which is well described by Stern-Volmer equation. The FITC-MIP can selectively and sensitively detect down to 0.0015 mg mL–1 of LYZ concentration. The excellent sensing performance of FITC-MIP suggests that FITC-MIP is a potential biosensor in clinical diagnosis applications.










Similar content being viewed by others
References
Akinalp AS, Asan M, Ozcan N. Expression of T4 lysozyme gene (gene e) in Streptococcus salivarius subsp. Thermophilus. Afr J Biotechnol. 2007;6:963–6.
Li S, Mulloor JJ, Wang L, Ji Y, Mulloor CJ, Micic M, et al. Strong and selective adsorption of lysozyme on graphene oxide. ACS Appl Mater Interfaces. 2014;6:5704–12.
Ou SH, Wu MC, Chou TC, Liu CC. Polyacrylamide gels with electrostatic functional groups for the molecular imprinting of lysozyme. Anal Chim Acta. 2004;504:163–6.
Pascual R, Gee B, Finch S. Usefulness of serum lysozyme analysis in diagnosis and evaluation of sarcoidosis. N Engl J Med. 1973;289:1074–6.
Perillie PE, Kaplan SS, Lefkowitz E, Rogaway W, Finch SC. Studies of muramidase (lysozyme) in leukemia. J Am Med Assoc. 1968;203:317–22.
Osserman EF, Lawlor DP. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966;124:921–52.
Prockop DJ, Davidson WD. A study of urinary and serum lysozyme in patients with renal disease. N Engl J Med. 1964;270:269–74.
Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc Lond Ser B. 1922;93:306–17.
Newman J, Cacatian A, Josephson A, Tsang A. Spinal-fluid lysozyme in the diagnosis of central-nervous-system tumours. Lancet. 1974;304:756–7.
Ding Z, Annie Bligh SW, Tao L, Quan J, Nie H, Zhu L, et al. Molecularly imprinted polymer based on MWCNT-QDs as fluorescent biomimetic sensor for specific recognition of target protein. Mater Sci Eng C. 2015;48:469–79.
Rachkov A, McNiven S, El’skaya A, Yano K, Karube I. Fluorescence detection of β-estradiol using a molecularly imprinted polymer. Anal Chim Acta. 2000;405:23–9.
Zourob M. Recognition receptors in biosensors. New York: Springer Science Business Media; 2010.
Garcia R, Cabrita MJ, Costa Freitas AM. Application of molecularly imprinted polymers for the analysis of pesticide residues in food-A highly selective and innovative approach. Am J Anal Chem. 2011;2:16–25.
Fu G, He H, Chai Z, Chen H, Kong J, Wang Y, et al. Enhanced lysozyme imprinting over nanoparticles functionalized with carboxyl groups for noncovalent template sorption. Anal Chem. 2011;83:1431–6.
He H, Fu G, Wang Y, Chai Z, Jiang Y, Chen Z. Imprinting of protein over silica nanoparticles via surface graft copolymerization using low monomer concentration. Biosens Bioelectron. 2010;26:760–5.
Qian L, Hu X, Guan P, Wang D, Li J, Du C, et al. The effectively specific recognition of bovine serum albumin imprinted silica nanoparticles by utilizing a macromolecularly functional monomer to stabilize and imprint template. Anal Chim Acta. 2015;884:97–105.
Chen H, Yuan D, Li Y, Dong M, Chai Z, Kong J, et al. Silica nanoparticle supported molecularly imprinted polymer layers with varied degrees of crosslinking for lysozyme recognition. Anal Chim Acta. 2013;779:82–9.
Liu D, Yang Q, Jin S, Song Y, Gao J, Wang Y, et al. Core–shell molecularly imprinted polymer nanoparticles with assistant recognition polymer chains for effective recognition and enrichment of natural low-abundance protein. Acta Biomater. 2014;10:769–75.
Gai Q-Q, Qu F, Liu Z-J, Dai R-J, Zhang Y-K. Superparamagnetic lysozyme surface-imprinted polymer prepared by atom transfer radical polymerization and its application for protein separation. J Chromatogr A. 2010;1217:5035–42.
Jia X, Xu M, Wang Y, Ran D, Yang S, Zhang M. Polydopamine-based molecular imprinting on silica-modified magnetic nanoparticles for recognition and separation of bovine hemoglobin. Analyst. 2013;138:651–8.
Lee M-H, Thomas JL, Ho M-H, Yuan C, Lin H-Y. Synthesis of magnetic molecularly imprinted poly(ethylene-co-vinyl alcohol) nanoparticles and their uses in the extraction and sensing of target molecules in urine. ACS Appl Mater Interfaces. 2010;2:1729–36.
Li L, He X, Chen L, Zhang Y. Preparation of core-shell magnetic molecularly imprinted polymer nanoparticles for recognition of bovine hemoglobin. Chem Asian J. 2009;4:286–93.
Li Y, Yang H-H, You Q-H, Zhuang Z-X, Wang X-R. Protein recognition via surface molecularly imprinted polymer nanowires. Anal Chem. 2006;78:317–20.
Wang J, Gao L, Han D, Pan J, Qiu H, Li H, et al. Optical detection of λ-cyhalothrin by core-shell fluorescent molecularly imprinted polymers in chinese spirits. J Agric Food Chem. 2015;63:2392–9.
Sunayama H, Ooya T, Takeuchi T. Fluorescent protein recognition polymer thin films capable of selective signal transduction of target binding events prepared by molecular imprinting with a post-imprinting treatment. Biosens Bioelectron. 2010;26:458–62.
Zhao Y, Ma Y, Li H, Wang L. Composite QDs@MIP nanospheres for specific recognition and direct fluorescent quantification of pesticides in aqueous media. Anal Chem. 2012;84:386–95.
Wu X, Zhang Z, Li J, You H, Li Y, Chen L. Molecularly imprinted polymers-coated gold nanoclusters for fluorescent detection of bisphenol A. Sensors Actuators B: Chem. 2015;211:507–14.
Campbell PA, Canono BP, Drevets DA (2001) Measurement of bacterial ingestion and killing by macrophages. Current protocols in immunology. Hoboken NJ: John Wiley and Sons, Inc.; 2001.
Grunberg E, Cleeland R. Fluorescence and viability of proteus mirabilis stained directly with fluorescein isothiocyanate. J Bacteriol. 1966;92:23–7.
Miller JS, Quarles JM. Flow cytometric identification of microorganisms by dual staining with FITC and PI. Cytometry. 1990;11:667–75.
Kelly KA, Reynolds F, Weissleder R, Josephson L. Fluorescein isothiocyanate-hapten immunoassay for determination of peptide-cell interactions. Anal Biochem. 2004;330:181–5.
McClatchey KD. Clinical laboratory medicine. Philadelphia, PA: Lippincott Wiliams and Wilkins; 2002.
Huang J, Liu H, Men H, Zhai Y, Xi Q, Zhang Z, et al. Molecularly imprinted polymer coating with fluorescence on magnetic particle. Macromol Res. 2013;21:1021–8.
Chen L, Li J, Wang S, Lu W, Wu A, Choo J, et al. FITC functionalized magnetic core-shell Fe3O4/Ag hybrid nanoparticle for selective determination of molecular biothiols. Sensors Actuators B Chem. 2014;193:857–63.
Feng H, Wang N, Tran TT, Yuan L, Li J, Cai Q. Surface molecular imprinting on dye-(NH2)-SiO2 NPs for specific recognition and direct fluorescent quantification of perfluorooctane sulfonate. Sensors Actuators B Chem. 2014;195:266–73.
Stobiecka M, Chalupa A. Modulation of plasmon-enhanced resonance energy transfer to gold nanoparticles by protein survivin channeled-shell gating. J Phys Chem B. 2015;119:13227–35.
Stobiecka M. Novel plasmonic field-enhanced nanoassay for trace detection of proteins. Biosens Bioelectron. 2014;55:379–85.
Lim GW, Lim JK, Ahmad AL, Chan DJC. Molecularly imprinted polymer layers using Navicula sp. frustule as core material for selectively recognition of lysozyme. Chem Eng Res Des. 2015;101:2–14.
Fowler CE, Buchber C, Lebeau B, Patarin J, Delacôte C, Walcarius A. An aqueous route to organically functionalized silica diatom skeletons. Appl Surf Sci. 2007;253:5485–93.
Hildebrand M. Biological processing of nanostructured silica in diatoms. Prog Org Coat. 2003;47:256–66.
Yu Y, Addai-Mensah J, Losic D. Functionalized diatom silica microparticles for removal of mercury ions. Sci Technol Adv Mater. 2012;13:1–11.
Lemonas JF. Diatomite. Am Ceram Soc Bull. 1997;76:92–5.
Lim GW, Lim JK, Ahmad AL, Chan DJC. Influences of diatom frustule morphologies on protein adsorption behavior. J Appl Phycol. 2015;27:763–75.
Baumgärtel T, von Borczyskowski C, Graaf H. Selective surface modification of lithographic silicon oxide nanostructures by organofunctional silanes. Beilstein J Nanotechnol. 2013;4:218–26.
Bergmann NM (2005) Molecularly imprinted polyacrylamide polymers and copolymers with specific recognition for serum proteins. Ph.D. Dissertation, The University of Texas.
Pang S, Liu S, Su X. A novel fluorescence assay for the detection of hemoglobin based on the G-quadruplex/hemin complex. Talanta. 2014;118:118–22.
Tan L, Kang C, Xu S, Tang Y. Selective room temperature phosphorescence sensing of target protein using Mn-doped ZnS QDs-embedded molecularly imprinted polymer. Biosens Bioelectron. 2013;48:216–23.
Zhang C, Cui H, Cai J, Duan Y, Liu Y. Development of fluorescence sensing material based on CdSe/ZnS quantum dots and molecularly imprinted polymer for the detection of carbaryl in rice and chinese cabbage. J Agric Food Chem. 2015;63:4966–72.
Loucaides S, Behrends T, Van Cappellen P. Reactivity of biogenic silica: surface versus bulk charge density. Geochim Cosmochim Acta. 2010;74:517–30.
Tozak KÖ, Erzengin M, Sargın İ, Ünlü N. Sorption of DNA by diatomite-Zn (II) embedded supermacroporous monolithic p(HEMA) cryogels. EXCLI J. 2013;12:670–80.
Bergmann NM, Peppas NA. Configurational biomimetic imprinting for protein recognition: structural characteristics of recognitive hydrogels. Ind Eng Chem Res. 2008;47:9099–107.
Siyam T, Abd-Elatif ZH. Gamma radiation-induced preparation of poly(acrylamide-acrylic acid-dimethylaminoethylmethacrylate) as exchanger. J Macromol Sci. 1999;36:417–28.
Ghosh SK, Ali M, Chatterjee H. Studies on the interaction of fluorescein isothiocyanate and its sugar analogues with cetyltrimethylammonium bromide. Chem Phys Lett. 2013;561(562):147–52.
Jiang L, Li X, Liu L, Zhang Q. Cellular uptake mechanism and intracellular fate of hydrophobically modified pullulan nanoparticles. Int Jf Nanomed. 2013;8:1825–34.
Ma LY, Wang HY, Xie H, Xu LX. A long lifetime chemical sensor: study on fluorescence property of fluorescein isothiocyanate and preparation of pH chemical sensor. Spectrochim Acta A. 2004;60:1865–72.
Sjöback R, Nygren J, Kubista M. Absorption and fluorescence properties of fluorescein. Spectrochim Acta A. 1995;51:L7–21.
Harz S, Schimmelpfennig M, Tse Sum Bui B, Marchyk N, Haupt K, Feller K-H. Fluorescence optical spectrally resolved sensor based on molecularly imprinted polymers and microfluidics. Eng Life Sci. 2011;11:559–65.
Deng Q, Wu J, Zhai X, Fang G, Wang S. Highly selective fluorescent sensing of proteins based on a fluorescent molecularly imprinted nanosensor. Sensors. 2013;13:12994.
Verheyen E, Schillemans JP, van Wijk M, Demeniex M-A, Hennink WE, van Nostrum CF. Challenges for the effective molecular imprinting of proteins. Biomaterials. 2011;32:3008–20.
Acknowledgments
This work is supported by Postgraduate Research Grant Scheme (grant no. 8046003), FRGS grant (6071271), and Research University Grant (814209). G.W.L. is financially assisted by MyPhD scholarship from the Ministry of Higher Education of Malaysia. All authors are affiliated with Membrane Science and Technology cluster USM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 246 kb)
Rights and permissions
About this article
Cite this article
Lim, G.W., Lim, J.K., Ahmad, A.L. et al. Fluorescent molecularly imprinted polymer based on Navicula sp. frustules for optical detection of lysozyme. Anal Bioanal Chem 408, 2083–2093 (2016). https://doi.org/10.1007/s00216-015-9298-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9298-6