Abstract
Ultrafast affinity extraction and a two-dimensional high performance affinity chromatographic system were used to measure the free fractions for various drugs in serum and at typical therapeutic concentrations. Pooled samples of normal serum or serum from diabetic patients were utilized in this work. Several drug models (i.e., quinidine, diazepam, gliclazide, tolbutamide, and acetohexamide) were examined that represented a relatively wide range of therapeutic concentrations and affinities for human serum albumin (HSA). The two-dimensional system consisted of an HSA microcolumn for the extraction of a free drug fraction, followed by a larger HSA analytical column for the further separation and measurement of this fraction. Factors that were optimized in this method included the flow rates, column sizes, and column switching times that were employed. The final extraction times used for isolating the free drug fractions were 333–665 ms or less. The dissociation rate constants for several of the drugs with soluble HSA were measured during system optimization, giving results that agreed with reference values. In the final system, free drug fractions in the range of 0.7–9.5 % were measured and gave good agreement with values that were determined by ultrafiltration. Association equilibrium constants or global affinities were also estimated by this approach for the drugs with soluble HSA. The results for the two-dimensional system were obtained in 5–10 min or less and required only 1–5 μL of serum per injection. The same approach could be adapted for work with other drugs and proteins in clinical samples or for biomedical research.

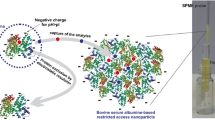
General scheme for the analysis of free drug fractions in serum by ultrafast affinity chromatography and two-dimensional affinity chromatography. The circles represent the drug and the half-circles on the left are serum proteins that can undergo reversible binding with this drug. The rectangles represent (from left-to-right) a guard column, an affinity microcolumn, and an analytical affinity column that are used to isolate and measure the drug's free fraction in serum





Similar content being viewed by others
References
Peters T Jr (1996) All about albumin: biochemistry, genetics and medical applications. Academic, San Diego, CA
Burtis CA, Ashwood ER, Bruns DE (2006) Tietz textbook of clinical chemistry and molecular diagnostics. Saunders, St Louis, MO
Zheng X, Li Z, Podariu MI, Hage DS (2014) Anal Chem 86:6454–6460
Hage DS, Jackson A, Sobansky MR, Schiel JE, Yoo MJ, Joseph KS (2009) J Sep Sci 32:835–853
Kwong TC (1985) Clin Chim Acta 151:193–216
Herve F, Urien S, Albengres E, Duche JC, Tillement JP (1994) Clin Pharmacokinet 26:44–58
Clarke W, Schiel JE, Moser A, Hage DS (2005) Anal Chem 77:1859–1866
Clarke W, Chowdhuri AR, Hage DS (2001) Anal Chem 73:2157–2164
Krebs HA (1950) Annu Rev Biochem 19:409–430
Zheng X, Yoo MJ, Hage DS (2013) Analyst 138:6262–6265
Zheng X, Matsuda R, Hage DS (2014) J Chromatogr A 1371:82–89
Melten JW, Wittebrood AJ, Hubb JJ, Faber GH, Wemer J, Faber DB (1985) J Pharm Sci 74:692–694
Musteata FM (2011) Bioanalysis 3:1753–1768
Matsuda R, Bi C, Anguizola J, Sobansky M, Rodriquez E, Badilla JV, Zheng X, Hage B, Hage DS (2014) J Chromatogr B 966:48–58
Vuignier K, Schappler J, Veuthey J, Carrupt P, Martel S (2010) Anal Bioanal Chem 398:53–66
Zheng X, Li Z, Beeram S, Podariu M, Matsuda R, Pfaunmiller EL, White CJ II, Carter N, Hage DS (2014) J Chromatogr B 968:49–63
Rich LR, Day YS, Morton TA, Myszka DG (2001) Anal Biochem 296:197–207
Dings F, Zhao G, Chen S, Liu F, Sun Y, Zhang L (2009) J Mol Struct 929:159–166
Leis D, Barbosa S, Attwood D, Taboada P, Mosquera V (2002) Langmuir 18:8178–8185
Yuan H, Pawliszyn J (2001) Anal Chem 73:4410–4416
Schiel JE, Hage DS (2009) J Sep Sci 32:1507–1522
Yoo MJ, Hage DS (2011) J Chromatogr A 1218:2072–2078
Schiel JE, Hage DS (2009) J Sep Sci 32:1507–1522
Mallik R, Yoo MJ, Briscoe CJ, Hage DS (2010) J Chromatogr A 1217:2796–2803
Ahene AB (2011) Bioanalysis 3:1287–1295
Holm SS, Hansen SH, Faber J, Staun-Olsen P (2004) Clin Biochem 37:85–93
Soldin OP, Soldin SJ (2011) Clin Biochem 44:89–94
Weber PC, Salemme FR (2003) Curr Opin Struct Biol 13:115–121
Zheng X, Bi C, Li Z, Podariu M, Hage DS (2015) J Pharm Biomed Anal. doi:10.1016/j.jpba.2015.01.042
Schiel JE, Tong Z, Sakulthaew C, Hage DS (2011) Anal Chem 83:9384–9390
Ohnmacht CM, Schiel JE, Hage DS (2006) Anal Chem 78:7547–7556
Anguizola J, Joseph KS, Barnaby OS, Matsuda R, Alvarado G, Clarke W, Cerny RL, Hage DS (2013) Anal Chem 85:4453–4460
Lee JW, Kim HJ, Kwon YS, Jun YH, Kim SK, Choi JW, Lee JE (2003) Ann Pediatr Endocrinol Metab 18:208–213
Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE (2002) Diabetes Care 25:275–278
Chen J, Hage DS (2006) Anal Chem 78:2672–2683
Joseph KS, Hage DS (2010) J Chromatogr B 878:1590–1598
Joseph KS, Anguizola J, Jackson AJ, Hage DS (2010) J Chromatogr B 878:2775–2781
Joseph KS, Anguizola J, Hage DS (2011) J Pharm Biomed Anal 54:426–432
Matsuda R, Anguizola J, Joseph KS, Hage DS (2011) Anal Bioanal Chem 401:2811–2819
Schiel JE (2009) Clinical and pharmaceutical applications of high-performance affinity chromatography. Ph.D. dissertation, University of Nebraska, Lincoln, NE
Regenthal R, Krueger M, Koeppel C, Preiss R (1999) J Clin Monit 15:529–544
Kratochwil NA, Huber W, Muller F, Kansy M, Gerber PR (2002) Biochem Pharmacol 64:1355–1374
Wilting J, Hart BJT, De Gier JJ (1980) Biochim Biophys Acta 626:291–298
Ueda CT, Makoid MC (1979) J Pharm Sci 68:448–450
Yoo MJ, Hage DS (2011) J Sep Sci 34:2255–2263
Acknowledgments
This work was supported by the National Institutes of Health under grants R01 GM044931 and R01 DK069629. M. Podariu was supported through a UCARE fellowship from the University of Nebraska-Lincoln. The concept of free fraction analysis by rapid affinity chromatography is the subject of U.S. Patent 6,720,193 (D.S. Hage and W.A. Clarke).
Conflict of interest
The authors declare that they have competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, X., Podariu, M., Matsuda, R. et al. Analysis of free drug fractions in human serum by ultrafast affinity extraction and two-dimensional affinity chromatography. Anal Bioanal Chem 408, 131–140 (2016). https://doi.org/10.1007/s00216-015-9082-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9082-7