Abstract
The inaccuracy of routine serum 25-hydroxyvitamin D measurements hampers the interpretation of data in patient care and public health research. We developed and validated a candidate reference measurement procedure (RMP) for highly accurate quantitation of two clinically important 25-hydroxyvitamin D metabolites in serum, 25-hydroxyvitamin D2 [25(OH)D2] and 25-hydroxyvitamin D3 [25(OH)D3]. The two compounds of interest together with spiked deuterium-labeled internal standards [d 3-25(OH)D2 and d 6-25(OH)D3] were extracted from serum via liquid-liquid extraction. The featured isotope-dilution LC-MS/MS method used reversed-phase chromatography and atmospheric pressure chemical ionization in positive ion mode. A pentafluorophenylpropyl-packed UHPLC column together with isocratic elution allowed for complete baseline resolution of 25(OH)D2 and 25(OH)D3 from their structural C-3 isomers within 12 min. We evaluated method trueness, precision, potential interferences, matrix effects, limits of quantitation, and measurement uncertainty. Calibration materials were, or were traceable to, NIST Standard Reference Materials 2972. Within-day and total imprecision (CV) averaged 1.9 and 2.0 % for 25(OH)D3, respectively, and 2.4 and 3.5 % for 25(OH)D2, respectively. Mean trueness was 100.3 % for 25(OH)D3 and 25(OH)D2. The limits of quantitation/limits of detection were 4.61/1.38 nmol/L for 25(OH)D3 and 1.46/0.13 nmol/L for 25(OH)D2. When we compared our RMP results to an established RMP using 40 serum samples, we found a nonsignificant mean bias of 0.2 % for total 25(OH)D. This candidate RMP for 25(OH)D metabolites meets predefined method performance specifications (≤5 % total CV and ≤1.7 % bias) and provides sufficient sample throughput to meet the needs of the Centers for Disease Control and Prevention Vitamin D Standardization Certification Program.

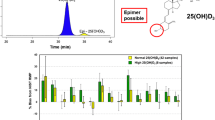
Bias assessment using NIST standard reference materials. Legend CDC mean mass fractions (ng/g) ± U 95 (6 replicates per mean). NIST-certified mass fractions (ng/g) ± U 95 from the Certificates of Analysis


Similar content being viewed by others
References
Cranney A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, Fang M, Yazdi F, Garritty C, Sampson M, Barrowman N, Tsertsvadze A, Mamaladze V (2007) Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 158:1–235
Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, Lichtenstein A, Patel K, Raman G, Tatsioni A, Terasawa T, Trikalinos TA (2009) Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep) 183:1–420
Wallace AM, Gibson S, de la Hunty A, Lamberg-Allardt C, Ashwell M (2010) Measurement of 25-hydroxyvitamin D in clinical laboratory: current procedures, performance characteristics and limitations. Steroids 75:477–488
Lind C, Chen J, Byrjalsen I (1997) Enzyme immunoassays for measuring 25-hydroxyvitamin D3 in serum. Clin Chem 43(6):943–949
Carter GD, Carter R, Jones J, Berry J (2004) How accurate are assays for 25-hydroxyvitamin D? Data from international vitamin D external quality assessment scheme. Clin Chem 50(11):2195–2197
Singh RJ, Taylor RL, Reddy S, Grebe S (2006) C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 91(8):3055–3061
Bedner M, Lippa KA, Tai SS (2013) An assessment of 25-hydroxyvitamin D measurements in comparability studies conducted by the vitamin D metabolites quality assurance program. Clin Chim Acta 426:6–11
Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK (2004) Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab 89(7):3152–3157
Tai S, Bedner M, Phinney K (2010) Development of candidate reference measurement procedure for determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 82:1942–1948
Stepman HCM, Vanderroost A, Van Uytfanghe K, Thienpont LM (2011) Candidate reference measurement procedure for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem 53(3):441–448
Phinney K, Bedner M, Tai S, Vamathevan V, Sander L, Sharpless K, Wise S, Yen JH, Schleicher RL, Chaudhary-Webb M, Pfeiffer CM, Betz JM, Coates PM, Picciano MF (2012) Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem 84(2):956–962
Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM (2012) Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Investig 72(Suppl 243):32–40
Thienpont LM, Stepman HC, Vesper HW (2012) Standardization of measurements of 25-hydroxyvitamin D3 and D2. Scand J Clin Lab Investig 72(Suppl 243):41–49
Cashman KD, Kiely M, Kinsella M, Durazo-Arvizu RA, Tian L, Zhang Y, Lucey A, Flynn A, Gibney MJ, Vesper HW, Phinney KW, Coates PM, Picciano MF, Sempos CT (2013) Evaluation of vitamin D standardization program protocols for standardizing serum 25-hydroxyvitamin D data: a case study of the program’s potential for national nutrition and health surveys. Am J Clin Nutr 97(6):1235–1242
Centers for Disease Control and Prevention. Laboratory quality assurance and standardization programs. CDC, Atlanta (GA). http://www.cdc.gov/labstandards/hs.html. Accessed 12 Nov 2014
Vesper HW, Botelho JC (2010) Standardization of testosterone measurements in humans. J Steroid Biochem Mol Biol 121(3–5):513–519
Vesper HW, Botelho JC (2012) An overview of CDC’s standardization initiative. Clin Lab News 38(6):10–12
Bureau International des Poids et Mesures. The international system of units (SI). http://www.bipm.org/en/about-us/. Accessed 12 Nov 2014
Vesper HW, Thienpont LM (2009) Traceability in laboratory medicine. Clin Chem 55(6):1067–1075
Stӧckl D, Sluss PM, Thienpont LM (2009) Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis. Clin Chim Acta 408:8–13
National Institute of Standards and Technology (2014) Certificate of analysis, standard reference material 2972: 25-Hydroxyvitamin D2 and D3 calibration solutions. NIST, Gaithersburg
Schleicher RL, Encisco SE, Chaudhary-Webb M, Paliakov EM, McCoy LF, Pfeiffer CM (2011) Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chim Acta 412:1594–1599
Taylor JK (1987) Quality assurance of chemical measurement. Lewis Publishers, Michigan
CLSI (2014) Preliminary evaluation of quantitative clinical laboratory measurement procedures; approved guideline-third edition. CLSI Document EP10-A3. CLSI, Wayne
ISO (2009) In vitro diagnostic medical devices-measurement of quantities in samples of biological origin-Requirements of content and presentation of reference measurement procedures. ISO document 15193:2009(E). ISO, Geneva
Antoni S, Vogl C. Release reagents for vitamin D compounds. US Patent 2013/0078729 A1, March, 28, 2013
Thienpont LM, Van Nieuwenhove B, Stöckl D, De Leenheer AP (1996) Calibration for isotope dilution mass spectrometry—description of an alternative to the bracketing procedure. J Mass Spectrom 31:1119–1125
Edwards SH, Kimberly MM, Pyatt SD, Stribling SL, Dobbin KD, Myers GL (2011) Proposed serum cholesterol reference measurement procedure by gas chromatography-isotope dilution mass spectrometry. Clin Chem 57(4):614–622
Grote-Koska D, Czajkowski S, Klauke R, Panten E, Brand K, Schumann G (2014) A candidate reference measurement procedure for cyclosporine A in whole blood. Accred Qual Assur 19:147–157
European Union Decision (2002) 2002/657/EC 17.08.2002: commission decision laying down performance criteria for the analytical methods to be used for certain substances and residues thereof in live animals and animal products. Off J Eur Communities 221:8–32
Society of Forensic Toxicology/American Academy of Forensic Science (2006) Forensic toxicology laboratory guidelines (2006). AAFS, Washington (DC). http://www.soft-tox.org/files/Guidelines_2006_Final.pdf. Accessed 12 Nov 2014
Food and Drug Administration (2003) Guidance for industry: mass spectrometry for confirmation of the identity of animal drug residues. FDA, Rockville (MD). http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM052658.pdf. Accessed 12 Nov 2014
Botelho JC, Shacklady C, Cooper HC, Tai S, Van Uytfanghe K, Thienpont LM, Vesper HW (2013) Isotope-dilution liquid chromatography mass spectrometry candidate reference method for total testosterone in human serum. Clin Chem 59(2):372–380
National Institute of Standards and Technology (2013) Certificate of analysis, standard reference material 972a: vitamin D metabolites in frozen human serum. NIST, Gaithersburg
National Institute of Standards and Technology (2013) Certificate of analysis, standard reference material 972: vitamin D in human serum. NIST, Gaithersburg
Acknowledgments
The authors thank Dr. Susan Tai from NIST for her guidance and advice during the method development. We would also like to thank Dr. Katleen Van Uytfanghe from the University of Ghent and Dr. Mary Bedner from NIST for their guidance on RMP measurement series acceptance criteria. In addition, we would like to thank all members of the hormone standardization research team at the CDC for providing logistic support.
Conflict of interest
No specific sources of financial support. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views or positions of the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 69 kb)
Rights and permissions
About this article
Cite this article
Mineva, E.M., Schleicher, R.L., Chaudhary-Webb, M. et al. A candidate reference measurement procedure for quantifying serum concentrations of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 407, 5615–5624 (2015). https://doi.org/10.1007/s00216-015-8733-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8733-z