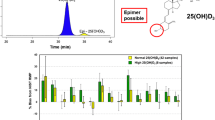
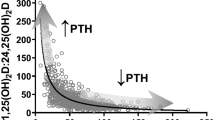
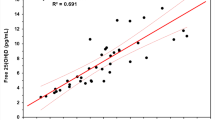
Abstract
An interlaboratory comparison study was conducted by the Vitamin D Standardization Program (VDSP) to assess the performance of ligand binding assays (Part 2) for the determination of serum total 25-hydroxyvitamin D [25(OH)D]. Fifty single-donor samples were assigned target values for concentrations of 25-hydroxyvitamin D2 [25(OH)D2], 25-hydroxyvitamin D3 [25(OH)D3], 3-epi-25-hydroxyvitamin D3 [3-epi-25(OH)D3], and 24R,25-dihydroxyvitamin D3 [24R,25(OH)2D3] using isotope dilution liquid chromatography–tandem mass spectrometry (ID LC-MS/MS). VDSP Intercomparison Study 2 Part 2 includes results from 17 laboratories using 32 ligand binding assays. Assay performance was evaluated using mean % bias compared to the assigned target values and using linear regression analysis of the test assay mean results and the target values. Only 50% of the ligand binding assays achieved the VDSP criterion of mean % bias ≤ |± 5%|. For the 13 unique ligand binding assays evaluated in this study, only 4 assays were consistently within ± 5% mean bias and 4 assays were consistently outside ± 5% mean bias regardless of the laboratory performing the assay. Based on multivariable regression analysis using the concentrations of individual vitamin D metabolites in the 50 single-donor samples, most assays underestimate 25(OH)D2 and several assays (Abbott, bioMérieux, DiaSorin, IDS-EIA, and IDS-iSYS) may have cross-reactivity from 24R,25(OH)2D3. The results of this interlaboratory study represent the most comprehensive comparison of 25(OH)D ligand binding assays published to date and is the only study to assess the impact of 24R,25(OH)2D3 content using results from a reference measurement procedure.
Graphical abstract





Similar content being viewed by others
References
Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM, VDSP. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest. 2012;72:32–40. https://doi.org/10.3109/00365513.2012.681935.
Binkley N, Dawson-Hughes B, Durazo-Arvizu R, Thamm M, Tian L, Merkel JM, et al. Vitamin D measurement standardization: the way out of the chaos. J Steroid Biochem Mol Biol. 2017;173:117–21. https://doi.org/10.1016/j.jsbmb.2016.12.002.
Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89(7):3152–7. https://doi.org/10.1210/jc.2003-031979.
Wise SA, Phinney KW, Tai SSC, Camara JE, Myers GL, Durazo-Arvizu R, et al. Baseline assessment of 25-hydroxyvitamin D assay performance: a Vitamin D Standardization Program (VDSP) interlaboratory comparison study. J AOAC Int. 2017;100(5):1244–52. https://doi.org/10.5740/jaoacint.17-0258.
Sempos CT, Binkley N. 25-Hydroxvitamin D assay standardization and vitamin D guidelines paralysis. Public Health Nutrition. 2020;23(7):1153–64. https://doi.org/10.1017/S1368980019005251.
Altieri B, Cavalier E, Bhattoa HP, Perez-Lopez FR, Lopez-Baena MT, Perez-Roncero GR, et al. Vitamin D testing: advantages and limits of the current assays. European Journal of Clinical Nutrition. 2020;74(2):231–47. https://doi.org/10.1038/s41430-019-0553-3.
Bivona G, Lo Sasso B, Iacolino G, Gambino CM, Scazzone C, Agnello L, et al. Standardized measurement of circulating vitamin D 25(OH)D and its putative role as a serum biomarker in Alzheimer’s disease and Parkinson’s disease. Clin Chim Acta. 2019;497:82–7. https://doi.org/10.1016/j.cca.2019.07.022.
Bjerg LN, Halgreen JR, Hansen SH, Morris HA, Jorgensen NR. An evaluation of total 25-hydroxyvitamin D assay standardization: where are we today? J Steroid Biochem Mol Biol. 2019;190:224–33. https://doi.org/10.1016/j.jsbmb.2019.03.015.
Fraser WD, Tang JCY, Dutton JJ, Schoenmakers I. Vitamin D measurement, the debates continue, new analytes have emerged, developments have variable outcomes. Calcified Tissue International. 2020;106(1):3–13. https://doi.org/10.1007/s00223-019-00620-2.
Herrmann M, Farrell CJL, Pusceddu I, Fabregat-Cabello N, Cavalier E. Assessment of vitamin D status - a changing landscape. Clin Chem Lab Med. 2017;55(1):3–26. https://doi.org/10.1515/cclm-2016-0264.
Makris K, Sempos C, Cavalier E. The measurement of vitamin D metabolites: part I-metabolism of vitamin D and the measurement of 25-hydroxyvitamin D. Horm-Int J Endocrinol Metab. 2020;19(2):81–96. https://doi.org/10.1007/s42000-019-00169-7.
Stokes CS, Lammert F, Volmer DA. Analytical methods for quantification of vitamin D and implications for research and clinical practice. Anticancer Res. 2018;38(2):1137–44. https://doi.org/10.21873/anticanres.12332.
Wise SA, Camara JE, Sempos CT, Burdette CQ, Hahm G, Nalin F, et al. (2022) Interlaboratory comparison of 25-hydroxyvitamin D assays: Vitamin D Standardization Program (VDSP) intercomparison study 2 – part 1 liquid chromatography – tandem mass spectrometry (LC-MS/MS) assays – impact of 3-epi-25-hydroxyvitamin D3 on assay performance. Anal Bioanal Chem, doi: 10.1007/s00216-021-3576-1
Phinney KW, Bedner M, Tai SSC, Vamathevan VV, Sander LC, Sharpless KE, et al. Development and certification of a Standard Reference Material for vitamin D metabolites in human serum. Anal Chem. 2012;84(2):956–62. https://doi.org/10.1021/ac202047n.
Phinney KW, Sempos CT, Tai SSC, Camara JE, Wise SA, Eckfeldt JH, et al. Baseline assessment of 25-hydroxyvitamin D reference material and proficiency testing/external quality assurance material commutability: a Vitamin D Standardization Program Study. J AOAC Int. 2017;100(5):1288–93. https://doi.org/10.5740/jaoacint.17-0291.
Tai SSC, Nelson MA, Bedner M, Lang BE, Phinney KW, Sander LC, et al. Development of Standard Reference Material (SRM) 2973 vitamin D metabolites in frozen human serum (high level). J AOAC Int. 2017;100(5):1294–303. https://doi.org/10.5740/jaoacint.17-0182.
Binkley N, Sempos CT, VDSP. Standardizing vitamin D assays: the way forward. J Bone Miner Res. 2014;29(8):1709–14. https://doi.org/10.1002/jbmr.2252.
Stockl D, Sluss PM, Thienpont LM. Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis. Clin Chim Acta. 2009;408(1-2):8–13. https://doi.org/10.1016/j.cca.2009.06.027.
Depreter B, Heijboer AC, Langlois MR. Accuracy of three automated 25-hydroxyvitamin D assays in hemodialysis patients. Clin Chim Acta. 2013;415:255–60. https://doi.org/10.1016/j.cca.2012.10.056.
Heijboer AC, Blankenstein MA, Kema IP, Buijs MM. Accuracy of 6 routine 25-hydroxyvitamin D assays: influence of vitamin D binding protein concentration. Clin Chem. 2012;58(3):543–8. https://doi.org/10.1373/clinchem.2011.176545.
Cavalier E, Lukas P, Bekaert AC, Carlisi A, Le Goff C, Delanaye P, et al. Analytical and clinical validation of the new Abbot Architect 25(OH) D assay: fit for purpose? Clin Chem Lab Med. 2017;55(3):378–84. https://doi.org/10.1515/cclm-2016-0566.
Cavalier E, Lukas P, Bekaert AC, Peeters S, Le Goff C, Yayo E, et al. Analytical and clinical evaluation of the new Fujirebio Lumipulse (R) G non-competitive assay for 25(OH)-vitamin D and three immunoassays for 25(OH) D in healthy subjects, osteoporotic patients, third trimester pregnant women, healthy African subjects, hemodialyzed and intensive care patients. Clin Chem Lab Med. 2016;54(8):1347–55. https://doi.org/10.1515/cclm-2015-0923.
Cavalier E, Lukas P, Crine Y, Peeters S, Carlisi A, Le Goff C, et al. Evaluation of automated immunoassays for 25(OH)-vitamin D determination in different critical populations before and after standardization of the assays. Clin Chim Acta. 2014;431:60–5. https://doi.org/10.1016/j.cca.2014.01.026.
Cavalier E, Rousselle O, Ferrante N, Carlisi A, Le Goff C, Souberbielle JC. Technical and clinical evaluation of the VITROS (R) Immunodiagnostic Products 25-OH Vitamin D Total Assay - comparison with marketed automated immunoassays and a liquid chromatography-tandem mass spectrometry method. Clin Chem Lab Med. 2013;51(10):1983–9. https://doi.org/10.1515/cclm-2013-0138.
Moreau E, Bacher S, Mery S, Le Goff C, Piga N, Vogeser M, et al. Performance characteristics of the VIDAS (R) 25-OH Vitamin D Total assay - comparison with four immunoassays and two liquid chromatography-tandem mass spectrometry methods in a multicentric study. Clin Chem Lab Med. 2016;54(1):45–53. https://doi.org/10.1515/cclm-2014-1249.
Elsenberg E, ten Boekel E, Huijgen H, Heijboer AC. Standardization of automated 25-hydroxyvitamin D assays: how successful is it? Clin Biochem. 2017;50(18):1126–30. https://doi.org/10.1016/j.clinbiochem.2017.06.011.
Hutchinson K, Healy M, Crowley V, Loew M. Verification of Abbott 25-(OH)-vitamin D assay on the architect system. Practical Laboratory Medicine. 2017;7:27–35. https://doi.org/10.1016/j.plabm.2017.01.001.
Annema W, Nowak A, von Eckardstein A, Saleh L. Evaluation of the new restandardized Abbott Architect 25-OH Vitamin D assay in vitamin D-insufficient and vitamin D-supplemented individuals. J Clin Lab Anal. 2018;32(4). https://doi.org/10.1002/jcla.22328.
Garnett E, Li J, Rajapakshe D, Tam E, Meng QH, Devaraj S. Efficacy of two vitamin D immunoassays to detect 25-OH vitamin D2 and D3. Practical Laboratory Medicine. 2019;17:e00130. https://doi.org/10.1016/j.plabm.2019.e00130.
Lim YK, Park AJ, Kweon OJ, Choi JH. Performance evaluation and measurement uncertainty determination of the new version of the Abbott Architect 25-OH Vitamin D 5P02 Assay. Am J Clin Pathol. 2019;151(2):209–16. https://doi.org/10.1093/ajcp/aqy131.
Camara J, Hoofnagle A, Carter G, Sempos C (2015) Take two: gearing up for the next vitamin D commutability study. Clinical Laboratory News (February 1, 2015)
Tai SSC, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2010;82(5):1942–8. https://doi.org/10.1021/ac9026862.
Tai SSC, Nelson MA. Candidate reference measurement procedure for the determination of (24R),25-dihydroxyvitamin D3 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2015;87(15):7964–70. https://doi.org/10.1021/acs.analchem.5b01861.
Wise SA, Camara JE, Sempos CT, Lukas P, Le Goff C, Peeters S, et al. Vitamin D Standardization Program (VDSP) intralaboratory study for the assessment of 25-hydroxyvitamin D assay performance. J Steroid Biochem Mol Biol. 2021;212:105917. https://doi.org/10.1016/j.jsbmb.2021.105917.
Carter GD, Berry J, Durazo-Arvizu R, Gunter E, Jones G, Jones J, et al. Hydroxyvitamin D assays: an historical perspective from DEQAS. J Steroid Biochem Mol Biol. 2018;177:30–5. https://doi.org/10.1016/j.jsbmb.2017.07.018.
Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104(2):454–61. https://doi.org/10.3945/ajcn.115.127985.
Schleicher RL, Sternberg MR, Looker AC, Yetley EA, Lacher DA, Sempos CT, et al. National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US population during 2007-2010. J Nutr. 2016;146(5):1051–61. https://doi.org/10.3945/jn.115.227728.
Cavalier E, Wallace AM, Carlisi A, Chapelle JP, Delanaye P, Souberbielle JC. Cross-reactivity of 25-hydroxy vitamin D2 from different commercial immunoassays for 25-hydroxy vitamin D: an evaluation without spiked samples. Clin Chem Lab Med. 2011;49(3):555–8. https://doi.org/10.1515/cclm.2011.072.
Cashman KD, Hayes A, Galvin K, Merkel J, Jones G, Kaufmann M, et al. Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: a double-edged sword? Clin Chem. 2015;61(4):636–45. https://doi.org/10.1373/clinchem.2014.234955.
Wyness SP, Straseski JA. Performance characteristics of six automated 25-hydroxyvitamin D assays: mind your 3s and 2s. Clin Biochem. 2015;48(16-17):1089–96. https://doi.org/10.1016/j.clinbiochem.2015.08.005.
Black LJ, Anderson D, Clarke MW, Ponsonby AL, Lucas RM, Ausimmune Investigator G. Analytical bias in the measurement of serum 25-hydroxyvitamin D concentrations impairs assessment of vitamin D status in clinical and research settings. PLoS One. 2015;10(8). https://doi.org/10.1371/journal.pone.0135478.
de Koning L, Al-Turkmani MR, Berg AH, Shkreta A, Law T, Kellogg MD. Variation in clinical vitamin D status by DiaSorin Liaison and LC-MS/MS in the presence of elevated 25-OH vitamin D-2. Clin Chim Acta. 2013;415:54–8. https://doi.org/10.1016/j.cca.2012.09.002.
Stepman HCM, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57(3):441–8. https://doi.org/10.1373/clinchem.2010.152553.
Denimal D, Roux S, Duvillard L. Evaluation of the new restandardized 25-hydroxyvitamin D assay on the iSYS platform. Clin Biochem. 2018;52:156–60. https://doi.org/10.1016/j.clinbiochem.2017.11.011.
Acknowledgements
The authors acknowledge David L. Duewer (NIST) for his suggestions and discussions regarding multivariable linear regression analysis. Bruno Emanuelli and Angelo Maggio (Care S.r.l), Vincent Chen and Jinyun Yuan (SNIBE), and Manisha Patwardhan (Golwilkar Metropolis Health Services Pvt. Ltd.) are acknowledged for contributing results to this study.
Funding
The Office of Dietary Supplements at the National Institutes of Health (NIH-ODS) provided partial funding for this study to the National Institute of Standards and Technology (NIST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The National Institute of Standards and Technology Research Protections Office reviewed the protocol for this project and determined that it is “not human subjects research” as defined in 15 CFR 27, the Common Rule for the Protection of Human Subjects.
Consent for publication
The laboratory study participants agreed to the publication of their measurement data, laboratory identification, and measurement assay platform identification.
Conflict of interest
S.A. Wise is an Editor of the journal Analytical and Bioanalytical Chemistry and was not involved in peer reviewing this manuscript. Several of the coauthors are employees of companies that produce assays that were evaluated in this study. There are no financial or nonfinancial competing interests for any of the coauthors.
Disclaimer
Certain commercial equipment or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology or the National Institutes of Health, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection celebrating ABCs 20th Anniversary.
Rights and permissions
About this article
Cite this article
Wise, S.A., Camara, J.E., Burdette, C.Q. et al. Interlaboratory comparison of 25-hydroxyvitamin D assays: Vitamin D Standardization Program (VDSP) Intercomparison Study 2 — Part 2 ligand binding assays — impact of 25-hydroxyvitamin D2 and 24R,25-dihydroxyvitamin D3 on assay performance. Anal Bioanal Chem 414, 351–366 (2022). https://doi.org/10.1007/s00216-021-03577-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03577-0