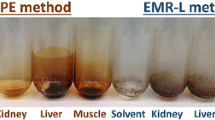
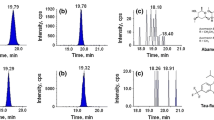
Abstract
Multiclass, multiresidue methods are becoming increasingly popular in regulatory monitoring programs due to their increased analytical scope and laboratory efficiency. In this work, we report the development and validation of a new high-throughput analytical method to monitor up to 131 veterinary drug residues, representing at least 13 different classes, in bovine muscle. This novel method streamlined sample preparation to <15 min/sample/analyst, or a batch of 40–60 pre-homogenized samples in <3 h/analyst, through the combination of dispersive solid-phase extraction with in-vial filtration (a new technique known as filter-vial d-SPE). The use of an enhanced sensitivity state-of-the-art tandem mass spectrometer led to <10 ng/g limits of quantification for nearly all drug analytes with injection of 0.17 mg of equivalent sample. Positive and negative switching in electrospray ionization was applied to cover all analytes in an 11-min liquid chromatographic separation. In the 3-day validation study, 100 of the drugs met quantification criteria of 70–120 % recoveries and Horwitz Ratio ≤1.0, and the remaining analytes could still be screened at regulatory target levels. In the validation study involving >11,400 analyte results for spiked samples, the rate of false negatives for identification purposes was <5 %, and no false positives occurred at appreciable concentrations.

The method of analysis





Similar content being viewed by others
References
USDA Food Safety and Inspection Service (2013) 2011 Residue sample results (www.fsis.usda.gov/wps/wcm/connect/f511ad0e-d148-4bec-95c7-22774e731f7c/2011_Red_Book.pdf?MOD=AJPERES). Accessed Sept 2014
USDA Foreign Agricultural Service (2014) Veterinary drug MRL database (www.mrldatabase.com/default.cfm?selectvetdrug=1). Accessed Sept 2014
USDA Food Safety and Inspection Service (2014) Screening and confirmation of animal drug residues by UHPLC-MS-MS. CLG-MRM1.04 (www.fsis.usda.gov/wps/wcm/connect/b9d45c8b-74d4-4e99-8eda-5453812eb237/CLG-MRM1.pdf?MOD=AJPERES). Accessed Sept 2014
Lehotay SJ, Lightfield AR, Geis-Asteggiante L, Schneider MJ, Dutko T, Ng C, Bluhm L, Mastovska K (2012) Development and validation of a streamlined method designed to detect residues of 62 veterinary drugs in bovine kidney using ultra-high performance liquid chromatography–tandem mass spectrometry. Drug Test Anal 4(Suppl 1):75–90
Geis-Asteggiante L, Lehotay SJ, Lightfield AR, Dutko T, Ng C, Bluhm L (2012) Ruggedness testing and validation of a practical analytical method for >100 veterinary drug residues in bovine muscle by ultrahigh performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1258:43–54
Schneider MJ, Lehotay SJ, Lightfield AR (2012) Evaluation of a multi-class, multi-residue liquid chromatography-tandem mass spectrometry method for analysis of 120 veterinary drugs in bovine kidney. Drug Test Anal 4(Suppl 1):91–102
Sporri AS, Jan P, Cognard E, Ortelli D, Edder P (2014) Comprehensive screening of veterinary drugs in honey by ultra-high-performance liquid chromatography coupled to mass spectrometry. Food Addit Contam A 34:806–816
Zhan J, Xu D-M, Sun J, Xu Y-J, Ni M-L, Yin J-Y, Chen J, Yu X-J, Huang Z-Q (2013) Comprehensive screening for multi-class veterinary drug residues and other contaminants in muscle using column-switching UPLC-MS/MS. Food Addit Contam A 30:1888–1899
Robert C, Gillard N, Brasseur P-Y, Pierret G, Ralet N, Dubois M, Delahaut P (2013) Rapid multi-residue and multi-class qualitative screening for veterinary drugs in foods of animal origin by UHPLC-MS/MS. Food Addit Contam A 30:443–457
Deng X-J, Yang H-Q, Li J-Z, Song Y, Guo D-H, Luo Y, Du X-N, Bo T (2011) Multiclass residues screening of 105 veterinary drugs in meat, milk, and egg using ultra high performance liquid chromatography tandem quadrupole time-of-flight mass spectrometry. J Liq Chromatogr Rel Technol 34:2286–2303
Biselli S, Schwalb U, Meyer A, Hartig L (2013) A multi-class, multi-analyte method for routine analysis of 84 veterinary drugs in chicken muscle using simple extraction and LC-MS/MS. Food Addit Contam A 30:921–939
Aguilera-Luiz MM, Romero-González R, Plaza-Bolaños P, Martínez-Vidal JL, Garrido-Frenich A (2013) Wide-scope analysis of veterinary drug and pesticide residues in animal feed by liquid chromatography coupled to quadrupole-time-of-flight mass spectrometry. Anal Bioanal Chem 405:6543–6553
Han L, Sapozhnikova Y, Lehotay SJ (2014) Streamlined sample cleanup using combined dispersive solid-phase extraction and in-vial filtration for analysis of pesticides and environmental pollutants in shrimp. Anal Chim Acta 827:40–46
USDA Food Composition Database (http://ndb.nal.usda.gov/ndb/). Accessed Sept 2014
Schneider MJ, Mastovska K, Lehotay SJ, Lightfield AR, Kinsella B, Shultz CE (2009) Comparison of screening methods for antibiotics in beef kidney juice and serum. Anal Chim Acta 637:290–297
Samanidou VF, Karageorgou EG (2011) On the use of Kinetex™-C18 core-shell 2.6 μm stationary phase to multiclass determination of antibiotics. Drug Test Anal 3:234–244
Stahnke H, Kittlaus S, Kempe G, Alder L (2012) Reduction of matrix effects in liquid chromatography-electrospray ionization-mass spectrometry by dilution of the sample extracts: how much dilution is needed? Anal Chem 84:1474–1482
Morel-Salmi C, Julia A, Vigor C, Vercauteren J (2014) A huge PVDF adsorption difference between resveratrol and ε-viniferin allows to quantitatively purify them and to assess their anti-tyrosinase property. Chromatographia 77:957–961
Horwitz W, Albert R (2006) The Horwitz ratio (HorRat): a useful index of method performance with respect to precision. J AOAC Int 89:1095–1109
Feng S, Chiesa OA, Kijak P, Chattopadhaya C, Lancaster V, Smith EA, Girard L, Sklenka S, Li H (2014) Determination of ceftiofur metabolite desfuroylceftiofur cysteine disulfide in bovine tissues using liquid chromatography–tandem mass spectrometry as a surrogate marker residue for ceftiofur. J Agric Food Chem 62:5011–5019
Anderson CA, Rupp HS, Wu WH (2005) Complexities in tetracycline analysis—chemistry, matrix extraction, cleanup, and liquid chromatography. J Chromatogr A 1075:23–32
Bottemiller H (2012) Codex adopts ractopamine limits for beef and pork—contentious 69–67 vote on key trade issue pits United States against China and the EU. Food Safety News (www.foodsafetynews.com/2012/07/codex-votes-69-67-to-advance-ractopamine-limits-for-beef-and-pork/#.U_z5Ybl0xD8). Accessed Sept 2014
U.S. Food and Drug Administration (2003) Guideline for industry: mass spectrometry for confirmation of the identity of animal drug residues. Fed Regist 68:25617–25618, www.fda.gov/cvm/guidance/guide118.pdf. Accessed Sept 2014
European Commission, Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed. SANCO/12571/2013 (www.eurl-pesticides.eu/). Accessed Sept 2014
Geis-Asteggiante L, Nunez A, Lehotay SJ, Lightfield AR (2014) Structural characterization of product ions by electrospray ionization and quadrupole time-of-flight mass spectrometry to support regulatory analysis of veterinary drug residues in foods. Rap Commun Mass Spectrom 28:1061–1081
Acknowledgments
AB Sciex is acknowledged for loan of the 6500 QTrap instrument, and Thompson Instrument Co. provided filter vials for evaluation. We thank John Phillips for providing statistical expertise and Robyn Moten, Bun-Hong Lai, and Limei Yun for their technical assistance in the laboratory.
Conflict of interest
Mention of brand or firm name does not constitute an endorsement by the US Department of Agriculture or the US Food and Drug Administration above others of a similar nature not mentioned. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection on Hormone and Veterinary Drug Residue Analysis with guest editors Siska Croubels, Els Daeseleire, Sarah De Saeger, Peter Van Eenoo, and Lynn Vanhaecke.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 70 kb)
Rights and permissions
About this article
Cite this article
Schneider, M.J., Lehotay, S.J. & Lightfield, A.R. Validation of a streamlined multiclass, multiresidue method for determination of veterinary drug residues in bovine muscle by liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 407, 4423–4435 (2015). https://doi.org/10.1007/s00216-014-8386-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8386-3