Abstract
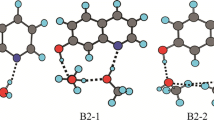
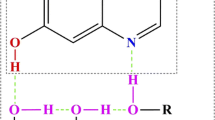
The reaction dynamics of excited-state intramolecular proton transfer (ESIPT) tautomerism in 10-hydroxybenzoquinoline (HBQ), 1-hydroxyanthraquinone (HAQ), methyl salicylate (MS) and 4-methyl-2,6-diformyl phenol (MFOH) has been investigated by quantum mechanics/molecular mechanics–molecular dynamics (QM/MM–MD) simulation with four different solvents H2O, CH3OH, CH2Cl2 and CHCl3. The ESIPT of HBQ proceeds 20 ± 8 fs in water and methanol solvents; whereas, for other two solvents, CH2Cl2 and CHCl3, proton transfer (PT) occurs within 62 ± 6 fs and 76 ± 6 fs, respectively. Similarly, the ESIPT of HAQ proceeds 21 ± 6 fs in H2O, 33 ± 6 fs in CH3OH, 51 ± 6 fs in CH2Cl2 and 61 ± 6 fs in CHCl3. The ESIPT of MS proceeds in 23 ± 6 fs in H2O, 38 ± 6 fs in CH3OH, 60 ± 6 fs in CH2Cl2 and 73 ± 6 fs in CHCl3. The ESIPT of MFOH proceeds 20 ± 6, 22 ± 6, 35 ± 6 and 58 ± 6 fs in H2O, CH3OH, CH2Cl2 and CHCl3 solvent, respectively. Our QM/MM–MD simulations show that the rate of PT is inversely proportional to the polarity of the solvents. In addition, intramolecular hydrogen bonding capacity between the hydroxyl oxygen (proton donor) and the benzoquinolinic nitrogen (proton acceptor) plays an important role for the ESIPT reaction.









Similar content being viewed by others
References
Kim CH, Joo T (2009) Coherent excited state intramolecular proton transfer probed by time-resolved fluorescence. Phys Chem Chem Phys 11:10266–10269
Piechowska J, Huttunen K, Wrobel Z, Lemmetyinen H, Tkachenko NV, Gryko DT (2012) Excited state intramolecular proton transfer in electron-rich and electron-poor derivatives of 10-hydroxybenzo[h]quinoline. J Phys Chem A 116:9614–9620
Chen KY, Hsieh CC, Cheng YM, Lai CH, Chou PT (2006) Extensive spectral tuning of the proton transfer emission from 550 to 675 nm via a rational derivatization of 10-hydroxybenzo[h]quinoline. Chem Commun 42:4395–4397
Higashi M, Saito S (2011) Direct simulation of excited-state intramolecular proton transfer and vibrational coherence of 10-hydroxybenzo[h]quinoline in solution. J Phys Chem Lett 2:2366–2371
Lee J, Kim CH, Joo T (2013) Active role of proton in excited state intramolecular proton transfer reaction. J Phys Chem A 117:1400–1405
Takeuchi S, Tahara T (2005) Coherent nuclear wavepacket motions in ultrafast excited-state intramolecular proton transfer: sub-30-fs resolved pump-probe absorption spectroscopy of 10-hydroxybenzo[h]quinoline in solution. J Phys Chem A 109:10199–10207
Lee J, Joo T (2014) Photophysical model of 10-hydroxybenzo[h]quinoline: internal conversion and excited state intramolecular proton transfer. Bull Korean Chem Soc 35:881–885
Chou PT, Chen YC, Yu WS, Chou YH, Wei CY, Cheng YM (2001) Excited-state intramolecular proton transfer in 10-hydroxybenzo[h]quinoline. J Phys Chem A 105:1731–1740
Fores M, Duran M, Sola M, Adamowicz L (1999) Excited-state intramolecular proton transfer and rotamerism of 2-(2′-hydroxyvinyl)benzimidazole and 2-(2′-hydroxyphenyl)imidazole. J Phys Chem A 103:4413–4420
Ryu J, Kim HW, Kim MS, Joo T (2013) Ultrafast excited state intramolecular proton transfer dynamics of 1-hydroxyanthraquinone in solution. Bull Korean Chem Soc 34:465–469
Douhal A, Sanz M, Carranza MA, Organero JA, Santos L (2004) Femtosecond observation of intramolecular charge- and proton-transfer reactions in a hydroxyflavone derivative. Chem Phys Lett 394:54–60
Mitra S, Tamai N, Mukherjee S (2006) Intramolecular proton transfer in 4-methyl-2,6-diformyl phenol and its derivative studied by femtosecond transient absorption spectroscopy. J Photochem Photobiol A 178:76–82
Tathe AB, Gupta VD, Shreykar MR, Ramasami P, Sekar N (2014) Excited state intramolecular proton transfer of 2-(2’,6’-dihydroxyphenyl)benzoxazole: insights using computational methods. J Lumin 154:267–273
Zhao J, Ji S, Chen Y, Guo H, Yang P (2012) Excited state intramolecular proton transfer (ESIPT): from principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys Chem Chem Phys 14:8803–8817
Douhal A, Sanz M, Tormo L, Organero JA (2005) Femtochemistry of inter- and intramolecular hydrogen bonds. ChemPhysChem 6:419–423
Stock K, Schriever C, Lochbrunner S, Riedle E (2008) Reaction path dependent coherent wavepacket dynamics in excited state intramolecular double proton transfer. Chem Phys 349:197–203
Lan X, Yang D, Sui X, Wang D (2013) Time-dependent density functional theory (TD-DFT) study on the excited-state intramolecular proton transfer (ESIPT) in 2-hydroxybenzoyl compounds: significance of the intramolecular hydrogen bonding. Spectrochim Acta Part A 102:281–285
Daeungern R, Kungwan N, Wolschann P, Aquino AJA, Lischka H, Barbatti M (2011) Excited-state intermolecular proton transfer reactions of 7-azaindole(MeOH)n (n=1-3) clusters in the gas phase: on-the-fly dynamics simulation. J Phys Chem A 115:14129–14136
Kyrychenko A, Waluk J (2006) Excited-state proton transfer through water bridges and structure of hydrogen-bonded complexes in 1H-pyrrolo[3,2-h]quinoline: adiabatic time-dependent density functional theory study. J Phys Chem A 110:11958–11967
Park SY, Jang DJ (2010) Accumulated proton-donating ability of solvent molecules in proton transfer. J Am Chem Soc 132:297–302
Stephan JS, Rodríguez CR, Grellmann KH, Zachariasse KA (1994) Flash-photolysis of 2-(2′-hydroxyphenyl)-3-H-indole. ground-state keto-enol tautomerization by mutual hydrogen exchange and by proton catalysis. Chem Phys 186:435–446
Jana S, Dalapati S, Guchhait N (2012) Proton transfer assisted charge transfer phenomena in photochromic schiff bases and effect of –NEt2 groups to the anil schiff bases. J Phys Chem A 116:10948–10958
Giordano L, Shvadchak VV, Fauerbach JA, Jares-Erijman EA, Jovin TM (2012) Highly solvatochromic 7-aryl-3-hydroxychromones. J Phys Chem Lett 3:1011–1016
Yan LW, Ye ZB, Peng CX, Zhang SH (2012) A new perylene diimide-based fluorescent chemosensor for selective detection of ATP in aqueous solution. Tetrahedron 68:2725–2727
Dong DP, Jing X, Zhang XL, Hu XY, Wu YY, Duan CY (2012) Gadolinium(III)-fluorescein complex as a dual modal probe for MRI and fluorescence zinc sensing. Tetrahedron 68:306–310
Schriever C, Lochbrunner S, Ofial AR, Riedle E (2011) The origin of ultrafast proton transfer: multidimensional wave packet motion vs. tunneling. Chem Phys Lett 503:61–65
Smith TP, Zaklika KA, Thakur K, Walker GC, Tominaga K, Barbara PF (1991) Spectroscopic studies of excited-state intramolecular proton transfer in 1-(acylamino)anthraquinones. J Phys Chem 95:10465–10475
Choi JR, Jeoung SC, Cho DW (2004) Two-photon-induced excited-state intramolecular proton transfer process in 1-hydroxyanthraquinone. Chem Phys Lett 385:384–388
Car R, Parrinello M (1985) Unified approach for molecular dynamics and density-functional theory. Phys Rev Lett 55:2471–2474
Uddin N, Kim J, Sung BJ, Choi TH, Choi CH, Kang H (2014) Comparative proton transfer efficiencies of hydronium and hydroxide in aqueous solution: proton transfer vs brownian motion. J Phys Chem B 118:13671–13678
Uddin N, Choi TH, Choi CH (2013) Direct absolute pKa predictions and proton transfer mechanisms of small molecules in aqueous solution by QM/MM-MD. J Phys Chem B 117:6269–6275
Ghosh MK, Uddin N, Choi CH (2012) Hydrophobic and hydrophilic associations of a methanol pair in aqueous solution. J Phys Chem B 116:14254–14260
Krylov AI (2001) Spin-flip configuration interaction: an electronic structure model that is both variational and size-consistent. Chem Phys Lett 350:522–530
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Lee C, Yang W, Parr RG (1988) Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Gordon MS, Slipchenko L, Li H, Jensen JH (2007) The effective fragment potential: a general method for predicting intermolecular interactions. Ann Rep Comput Chem 3:177–193
Dang LX, Chang TM (2003) Many-body interactions in liquid methanol and its liquid/vapor interface: a molecular dynamics study. J Chem Phys 119:9851–9857
Choi CH, Re S, Feig M, Sugita Y (2012) Quantum mechanical/effective fragment potential molecular dynamics (QM/EFP-MD) study on intra-molecular proton transfer of glycine in water. Chem Phys Lett 539–540:218–221
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Acknowledgements
We are grateful to Professor Cheol Ho Choi, Kyungpook National University, for his support to use his laboratory facilities for this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MAMR worked in investigation, methodology and writing—review and editing; TA helped in reviewing, validation and visualization; NU helped in conceptualization, supervision and writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rashid, M.A., Acter, T. & Uddin, N. Excited-state intramolecular proton transfer in 10-hydroxybenzoquinoline, 1-hydroxyanthraquinone, methyl salicylate and 4-methyl-2,6-diformyl phenol: a QM/MM–MD study. Theor Chem Acc 142, 96 (2023). https://doi.org/10.1007/s00214-023-03045-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03045-1