Abstract
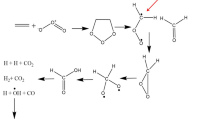
Proton transfer (PT) reactions of 7-hydroxyquinoline (7HQ) via intermolecular hydrogen-bonded wire of methanol, water and mixed methanol–water in ground (S0) and first excited (S1) states, [7HQ(X)3 when X = M-methanol and W-water], have been studied by using density functional theory (DFT) at B3LYP and its time-dependent DFT with 6-31+G(d,p) basis set. For all complexes, the intermolecular hydrogen bonds become shorter and the O–H stretching vibrational frequencies shift to lower frequencies in the S1 state, which confirm that the hydrogen bonding interaction is stronger in the S1 state. Moreover, the frontier molecular orbitals of all complexes were analyzed to confirm the PT reactions (ππ*). The simulated absorption and emission spectra of 7HQ(MMM) are in good agreement with the experimental data. In addition, the potential energy curves along the PT reaction coordinates of all complexes both in S0 and S1 states were scanned by constrained optimizations fixing the O–H bond distance (a proton donor site of 7HQ) to investigate the effect of different intermolecular hydrogen-bonded solvent wires surrounding 7HQ. PT reactions are found to be favorable in S1 state due to the low PT energy barrier. For pure solvent, the excited-stated proton transfer (ESPT) occurs faster via methanol than water. For mixed solvent, when replacing methanol with one up to three water molecules, PT energy barrier is found to be higher than that of 7HQ(MMM); therefore, water may block the ESPT reaction.







Similar content being viewed by others
References
Watson JD, Crick FHC (1953) Nature 171:737–738
Arnaut LG, Formosinho SJ (1993) J Photochem Photobiol A Chem 75:1–20
Formosinho SJ, Arnaut LG (1993) J Photochem Photobiol A Chem 75:21–48
Geissler PL, Dellago C, Chandler D, Hutter J, Parrinello M (2001) Science 291:2121–2124
Tanner C, Manca C, Leutwyler S (2003) Science 302:1736–1739
Tanner C, Thut M, Steinlin A, Manca C, Leutwyler S (2006) J Phys Chem A 110:1758–1766
Thut M, Tanner C, Steinlin A, Leutwyler S (2008) J Phys Chem A 112:5566–5572
Mason SF, Philp J, Smith BE (1968). J Chem Soc A 3051–3056
Itoh M, Adachi T, Tokumura K (1983) J Am Chem Soc 105:4828–4829
Thistlethwaite PJ (1983) Chem Phys Lett 96:509–512
Itoh M, Adachi T, Tokumura K (1984) J Am Chem Soc 106:850–855
Thistlethwaite PJ, Corkill PJ (1982) Chem Phys Lett 85:317–321
Nakagawa T, Kohtani S, Itoh M (1995) J Am Chem Soc 117:7952–7957
Kohtani S, Tagami A, Nakagaki R (2000) Chem Phys Lett 316:88–93
Park S-Y, Kim H-B, Yoo BK, Jang D-J (2012) J Phys Chem B 116:14153–14158
Matsumoto Y, Ebata T, Mikami N (2001) Chem Phys Lett 338:52–60
Matsumoto Y, Ebata T, Mikami N (2002) J Phys Chem A 106:5591–5599
Al-Lawatia N, Husband J, Steinbrecher T, Abou-Zied OK (2011) J Phys Chem A 115:4195–4201
Bekcioglu G, Allolio C, Ekimova M, Nibbering ETJ, Sebastiani D (2014) Phys Chem Chem Phys 16:13047–13051
Cui Y, Zhao H, Zhao J, Li P, Song P, Xia L (2015) New J Chem 39:9910–9917
Konijnenberg J, Ekelmans GB, Huizer AH, Varma CAGO (1989) J Chem Soc Faraday Trans 2(85):39–51
Kim T-G, Lee S-I, Jang D-J, Kim Y (1995) J Phys Chem 99:12698–12700
Lee S-I, Jang D-J (1995) J Phys Chem 99:7537–7541
Fang W-H (1998) J Am Chem Soc 120:7568–7576
Park S-Y, Kim B, Lee Y-S, Kwon O-H, Jang D-J (2009) Photochem Photobiol Sci 8:1611–1617
Park S-Y, Lee Y-S, Kwon O-H, Jang D-J (2009). Chem Commun 926–928
Lim H, Park S-Y, Jang D-J (2010) J Phys Chem A 114:11432–11435
Park S-Y, Jang D-J (2010) J Am Chem Soc 132:297–302
Kang B, Ko KC, Park S-Y, Jang D-J, Lee JY (2011) Phys Chem Chem Phys 13:6332–6339
Kwon O-H, Lee Y-S, Yoo BK, Jang D-J (2006) Angew Chem Int Ed 45:415–419
Fradelos G, Kaminski JW, Wesolowski TA, Leutwyler S (2009) J Phys Chem A 113:9766–9771
Bach A, Leutwyler S (1999) Chem Phys Lett 299:381–388
Kang B, Jang D-J, Lee JY (2015) Chem Phys 456:8–12
Daengngern R, Kerdpol K, Kungwan N, Hannongbua S, Barbatti M (2013) J Photochem Photobiol A Chem 266:28–36
Kerdpol K, Daengngern R, Kungwan N (2015) Mol Simul 41:1177–1186
Becke AD (1993) J Chem Phys 98:5648–5652
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) J Phys Chem 98:11623–11627
Corni S, Cammi R, Mennucci B, Tomasi J (2005) J Chem Phys 123:134512
Jacquemin D, Perpète EA, Scalmani G, Frisch MJ, Assfeld X, Ciofini I, Adamo C (2006) J Chem Phys 125:164324
Jacquemin D, Mennucci B, Adamo C (2011) Phys Chem Chem Phys 13:16987–16998
Grabowski SJ (2004) J Phys Org Chem 17:18–31
Ganguly A, Paul BK, Ghosh S, Guchhait N (2013) Comput Theor Chem 1018:102–114
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JJA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford, CT
Zhao G-J, Han K-L (2012) Acc Chem Res 45:404–413
Zhao G-J, Han K-L (2008) J Comput Chem 29:2010–2017
Zhao G-J, Han K-L (2010) In: Han K-L, Zhao G-J (eds) Hydrogen bonding and transfer in the excited state. Wiley, Chichester, pp 149–158
Savarese M, Netti PA, Rega N, Adamo C, Ciofini I (2014) Phys Chem Chem Phys 16:8661–8666
Acknowledgments
The authors wish to thank the Thailand Research Fund (RSA5880057) for financial support. K. Kerdpol thanks the Science Achievement Scholarship of Thailand (SAST), Faculty of Science, Chiang Mai University, Chiang Mai, Thailand. Moreover, the Graduate School, Chiang Mai University and National Nanotechnology Center (NANOTEC) of Thailand are also acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kerdpol, K., Daengngern, R., Meeprasert, J. et al. Theoretical insights into photoinduced proton transfer of 7-hydroxyquinoline via intermolecular hydrogen-bonded wire of mixed methanol and water. Theor Chem Acc 135, 208 (2016). https://doi.org/10.1007/s00214-016-1963-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1963-0