Abstract
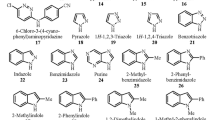
It is well known that the feasibility of electron transfer reactions and the thermodynamic relationship between radicals and anions are highly dependent on the standard redox potentials. However, due to experimental limitations on the measurement of irreversible redox reactions, it is often difficult or even impossible to obtain reliable oxidation or reduction potential data. In this study, we assessed the performance of different theoretical methods and solvation models for predicting standard oxidation potentials (\({E}_{\mathrm{ox}}^{\mathrm{o}}\)) of 33 representative anions including alcoholate, phenolate, and carboxylate anions in aprotic polar solvents. The density functional theory (DFT) M06-2X method and the composite procedure CBS-QB3 both achieved ideal precision for predicting adiabatic ionization potentials (IPs) in the gas phase, with a mean absolute deviation (MAD) value of 0.06 eV and 0.08 eV, respectively. As for solvation contributions, the SMD (solvation model density) model performed better than the latest uESE (Universal Easy Solvation Energy Evaluation) model for this kind of radical/anion couple. We suggest the optimal combination of M06-2X/ma-TZVP for IPs, and the SMD solvation model at M05-2X/6-31G(d) level is suitable for predicting \({E}_{\mathrm{ox}}^{\mathrm{o}}\), with an average error of 0.08 V. This assessment provides an alternative and reliable theoretical procedure to predict the oxidation potential and other related thermodynamic properties of O-centered organic anions.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Hammerich O, Speiser B (eds) (2016) Organic electrochemistry: revised and expanded, 5th edn. Boca Raton, CRC Press
Magri DC, Workentin MS (2012) Redox properties of radicals. In: Encyclopedia of radicals in chemistry, biology and materials
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA (1999) A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J Chem Phys 110(6):2822–2827
Marenich AV, Ho J, Coote ML, Cramer CJ, Truhlar DG (2014) Computational electrochemistry: prediction of liquid-phase reduction potentials. Phys Chem Chem Phys 16(29):15068–15106
Kohn W, Becke AD, Parr RG (1996) Density functional theory of electronic structure. J Phys Chem 100(31):12974–12980
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem 113(18):6378–6396
Arumugam K, Becker U (2014) Computational redox potential predictions: applications to inorganic and organic aqueous complexes, and complexes adsorbed to mineral surfaces. Minerals 4(2):345–387
Fu Y, Liu L, Wang Y-M, Li J-N, Yu T-Q, Guo Q-X (2006) Quantum-chemical predictions of redox potentials of organic anions in dimethyl sulfoxide and reevaluation of bond dissociation enthalpies measured by the electrochemical methods. J Phys Chem 110(17):5874–5886
Schmidtam Busch M, Knapp E-W (2005) One-Electron Reduction Potential for Oxygen- and Sulfur-Centered Organic Radicals in Protic and Aprotic Solvents. J Am Chem Soc 127(45):15730–15737
Guerard JJ, Arey JS (2013) Critical evaluation of implicit solvent models for predicting aqueous oxidation potentials of neutral organic compounds. J Chem Theory Comput 9(11):5046–5058
Männistö PT, Kaakkola S (1999) Catechol-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev 51(4):593
Isegawa M, Neese F, Pantazis DA (2016) Ionization energies and aqueous redox potentials of organic molecules: comparison of DFT, correlated ab initio theory and pair natural orbital approaches. J Chem Theory Comput 12(5):2272–2284
Isse AA, Gennaro A (2010) Absolute potential of the standard hydrogen electrode and the problem of interconversion of potentials in different solvents. J Phys Chem 114(23):7894–7899
Parker VDHKL, Roness F et al (1991) Electrode potentials and the thermodynamics of isodesmic reactions. J Am Chem Soc 113(20):7493–7498
Frisch MJTG, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 09, Revision E.02. Gaussian Inc, Wallingford CT
Lewars EG (2011) Computational Chemistry: Introduction to the Theory and Applications of Molecular and Quantum Mechanics, vol 318. Springer, New York
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27(15):1787–1799
Cossi M, Barone V, Cammi R, Tomasi J (1996) Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett 255(4):327–335
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120(1):215–241
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393(1):51–57
Chai J-D, Head-Gordon M (2008) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys 128(8):084106
Grimme S (2006) Semiempirical hybrid density functional with perturbative second-order correlation. J Chem Phys 124(3):034108
Psciuk BT, Lord RL, Munk BH, Schlegel HB (2012) Theoretical determination of one-electron oxidation potentials for nucleic acid bases. J Chem Theory Comput 8(12):5107–5123
Tu Y-J, Njus D, Schlegel HB (2017) A theoretical study of ascorbic acid oxidation and HOO/O2—radical scavenging. Org Biomol Chem 15(20):4417–4431
Xu L, Coote ML (2019) Methods to improve the calculations of solvation model density solvation free energies and associated aqueous pKa values: comparison between choosing an optimal theoretical level, solute cavity scaling, and using explicit solvent molecules. J Phys Chem 123(34):7430–7438
Zheng J, Xu X, Truhlar DG (2011) Minimally augmented Karlsruhe basis sets. Theor Chem Acc 128(3):295–305
Xu L, Coote ML (2019) Improving the accuracy of PCM-UAHF and PCM-UAKS calculations using optimized electrostatic scaling factors. J Chem Theory Comput 15(12):6958–6967
Vyboishchikov SF, Voityuk AA (2021) Fast non-iterative calculation of solvation energies for water and non-aqueous solvents. J Comput Chem 42(17):1184–1194
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Ho J, Ertem MZ (2016) Calculating free energy changes in continuum solvation models. J Phys Chem 120(7):1319–1329
Romańczyk PP, Rotko G, Kurek SS (2014) The redox potential of the phenyl radical/anion couple and the effect thereon of the lithium cation: a computational study. Electrochem Commun 48:21–23
Đorović J, Marković JMD, Stepanić V, Begović N, Amić D, Marković Z (2014) Influence of different free radicals on scavenging potency of gallic acid. J Mol Model 20(7):2345
Isegawa M, Truhlar DG (2013) Valence excitation energies of alkenes, carbonyl compounds, and azabenzenes by time-dependent density functional theory: linear response of the ground state compared to collinear and noncollinear spin-flip TDDFT with the Tamm–Dancoff approximation. J Chem Phys 138(13):134111
Isegawa M, Peverati R, Truhlar DG (2012) Performance of recent and high-performance approximate density functionals for time-dependent density functional theory calculations of valence and Rydberg electronic transition energies. J Chem Phys 137(24):244104
Vikramaditya T, Lin S-T (2017) Assessing the role of Hartree-Fock exchange, correlation energy and long range corrections in evaluating ionization potential, and electron affinity in density functional theory. J Comput Chem 38(21):1844–1852
Antonello S, Formaggio F, Moretto A, Toniolo C, Maran F (2001) Intramolecular, intermolecular, and heterogeneous nonadiabatic dissociative electron transfer to peresters. J Am Chem Soc 123(39):9577–9584
Hansch C, Leo A, Taft R (1991) A survey of Hammett substituent constants and resonance and field parameters. Chem Rev 91(2):165–195
Donkers RL, Maran F, Wayner DDM, Workentin MS (1999) Kinetics of the reduction of dialkyl peroxides. New insights into the dynamics of dissociative electron transfer1. J Am Chem Soc 121(31):7239–7248
Antonello S, Musumeci M, Wayner DDM, Maran F (1997) Electroreduction of dialkyl peroxides. Activation−driving force relationships and bond dissociation free energies. J Am Chem Soc 119(40):9541–9549
Bordwell FG, Singer DL, Satish AV (1993) Effects of structural changes on acidities and homolytic bond dissociation energies of the nitrogen-hydrogen bonds in pyridones and related heterocycles. J Am Chem Soc 115(9):3543–3547
Grampp G, Landgraf S, Mureşanu C (2004) Redox properties and bond dissociations energies of phenoxyl radicals. Electrochim Acta 49(4):537–544
Bordwell FG, Cheng J (1991) Substituent effects on the stabilities of phenoxyl radicals and the acidities of phenoxyl radical cations. J Am Chem Soc 113(5):1736–1743
Hapiot PPJ, Yousfi N (1992) Substituent effects on the redox properties of phenolates in acetonitrile. One-electron redox potentials New J Chem 16:877–877
Antonello S, Maran F (1999) The role and relevance of the transfer coefficient α in the study of dissociative electron transfers: concepts and examples from the electroreduction of perbenzoates. J Am Chem Soc 121(41):9668–9676
Zhao Y, Bordwell FG (1996) Bond dissociation free energies (BDFEs) of the acidic H−A bonds in HA•− radical anions by three different pathways. J Org Chem 61(19):6623–6626
Neugebauer H, Bohle F, Bursch M, Hansen A, Grimme S (2020) Benchmark study of electrochemical redox potentials calculated with semiempirical and DFT methods. J Phys Chem A 124(35):7166–7176
Acknowledgements
Financial support from National Natural Science Foundation of China (NSFC 21927814) and generous support by the School of Pharmaceutical Science and Technology, Tianjin University, China, including computer time on the SPST computer cluster Arran are gratefully acknowledged.
Funding
This work was supported by National Natural Science Foundation of China (NSFC 21927814).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. JZ designed the experiments. XW performed the experiments. XW and JZ analyzed the data and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests regarding the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Li, F. & Zhang, J. Theoretical prediction for redox potentials of oxygen-centered organic anions in aprotic solvents. Theor Chem Acc 142, 62 (2023). https://doi.org/10.1007/s00214-023-03002-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03002-y