Abstract
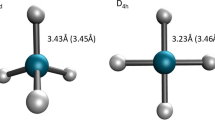
Our gargantuan ab initio all-electron fully relativistic Dirac–Fock (DF), nonrelativistic (NR) Hartree–Fock (HF) and Dirac–Fock-Breit-Gaunt (DFBG) molecular SCF calculations for the superheavy octahedral oganesson hexatenniside OgTs6 predict atomization energies (AEs) of 9.49, −5.54 and 9.37 eV, at the optimized Og-Ts bond distances of 3.35, 3.44 and 3.36 Å, respectively. There are dramatic effects of relativity for the atomization energy for OgTs6 (with seven superheavy atoms and 820 electrons) of ~15.0 eV at both the DF and DFBG levels of theory. Our calculated energies of reaction for the superheavy reaction Og + 3Ts2 → OgTs6 at the NR, DF and DFBG levels of the theory are exoergic by 8.81, 6.33 and 6.26 eV, respectively. The contribution to dispersion interactions increases the DFBG reaction energy for Og + 3Ts2 → OgTs6 by 0.94–7.20 eV. The AEs of OgTs6 to form Og + 6Ts are calculated to be −5.54 at the NR level of theory (that is, OgTs6 is unstable relative to the sum of the NR energies of its constituent atoms) and 9.49 and 9.37 eV at the DF and DFBG levels of theory, respectively. Dispersion interactions increase the AE(DFBG) by 1.30–10.67 eV. Ours are the first dispersion interaction corrected calculations for OgTs6 with seven superheavy atoms and 820 electrons. Mulliken population analysis (MPA) as implemented in the DIRAC code for our DF and NR calculations (using the dyall.cv4z basis) yields the charges Og+0.60 and Og+0.96, respectively, on the central Og atom indicating that our relativistic DF calculations predict octahedral OgTs6 to be less ionic compared to our NR HF calculations. However, due caution must be used to interpret the results of MPA, which are highly basis set dependent.
Similar content being viewed by others
References
Karol PJ, Barber RC, Sherrill BM, Vardaci E, Yamazaki T (2015) Pure App Chem 88:155
Conover E (2019) Science news, February 27 2019 (https://www.sciencenews.org/physics-periodic-table-future-superheavy-elements)
Oganessian YT, Dmitriev SN (2016) Russ Chem Rev 85:901
Kean S (2019) Science 363:466
Haba H et al (2007) Eur Phys J D 45:81
Van Noorden R (2016) Nature News & Comment. https://www.nature.com/news/four-chemical-elements-added-to-periodic-table-1.19112
Oganessian YT (2010) Phys Rev Lett 104:142502
Hofmann S (2009) Lecture Notes Phys 764:203
Gaggeler HW (2009) Russ Chem Rev 78:1139
Oganessian YT (2006) Pure Appl Chem 78:889
Malli GL, Oreg J (1975) J Chem Phys 63:830
Malli GL (1980) Chem Phys Lett 73:510
Malli GL (1983) Relativistic effects in ATOMS, molecules and solids. In: Malli GL (ed) NATO ASI SERIES B Physics. Plenum Press, New York, pp 183–211
Malli GL (1994) Relativistic and electron correlation effects in molecules and solids. In: Malli GL (ed) NATO ASI SERIES B, Physics. Plenum Press, New York, pp 1–15
Malli GL (1997) In proceedings of the Robert A. Welch foundation, 41st conference on chemical research THE TRANSACTINIDE ELEMENTS, pp 197–228, Houston, Texas. pp. 27–28
Malli GL, Styszynski J (1996) J Chem Phys 104:1012
Malli GL (1998) J Chem Phys 109:4448
Munoz-Castro A, Carey DM, Arratia-Perez R, Malli GL (2012) Polyhedron 39:113
Malli GL (2004). In: Brandas EJ, Kryachko ES (eds) Fundamental world of quantum chemistry, vol III. Kluwer Academic Press, Dordrecht, pp 323–363
Malli GL (2007) Theor Chem Acc 118:473
Malli GL (2015) J Chem Phys 142:064311
Malli GL (2016) J Chem Phys 144:194301
Malli GL (2006) J Chem Phys 124:071102
Visscher L, Visser O, Aerts PJC, Merenga H, Nieuwpoort WC (1994) Comput Phys Commun 81:120
DIRAC, a relativistic ab initio electronic structure program, Release DIRAC12 (2012), written by Aa. Jensen HJ, Bast R, Saue T, and Visscher L, with contributions from Bakken V, Dyall KG, Dubillard S, Ekstroem U, Eliav E, Enevoldsen T, Fleig T, Fossgaard O, Gomes ASP, Helgaker T, Laerdahl JK, Lee YS, Henriksson J, Ilias M, Ch. R. Jacob, Knecht S, Komorovsky S, Kullie O, Larsen CV, Nataraj HS, Norman P, Olejniczak G, Olsen J, Park YC, Pedersen JK, Pernpointner M, Ruud K, Salek P, Schimmelpfennig B, Sikkema J, Thorvaldsen AJ, Thyssen J, van Stralen J, Villaume S, Visser O, Winther T, and Yamamoto S (see http://www.diracprogram.org)
Stanton RE, Havriliak S (1984) J Chem Phys 82:1910
Breit G (1929) Phys Rev 34:553
Mulliken RS (1955) J Chem Phys 23:1833
Roby KR (1974) Mol Phys 47:81
Grimme S, Antony G, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
Becke AD, Johnson ER (2007) J Chem Phys 127:154108
Slater JC, Kirkwood JG (1931) Phys Rev 37:682
Stone A (2013) The theory of intermolecular forces. Oxford University Press, Oxford, p 68
Schwerdtfeger P, Nagle JK (2019) Mol Phys 117:1200
Tkatchenko A, Fedorov DV https://arxiv.org/pdf/2007.02992.pdf.
Rahm M, Hoffmann R, Ashcroft NW (2016) Chem Euro J 22:14625
Acknowledgements
This research is supported in part by the U.S. Department of Energy, Office of Science, Office of Nuclear Physics under Award No. DE-FG06-97ER 410266. This research has used in part resources of the National Energy Research Scientific Computing Center (NERSC), which is supported by the Office of Science of the U.S. Department of Energy under Contract No.DE-AC03-76SF00098. We gratefully acknowledge the superb NERSC facility which is a sine qua non for our gargantuan calculations. Part of our extensive calculations was carried out using the Westgrid computing resources at Simon Fraser University, Burnaby, BC, Canada which are gratefully acknowledged. Our sincerest thanks to the anonymous reviewers for their very useful and helpful comments, especially the Reviewer who suggested including the dispersion interaction corrections for OgTs6. GAD is grateful to Professor Erin R. Johnson for helpful discussions. We are most grateful to the Editors for their help and advice. We express our sincerest thanks to Prof. Tanmoy Chakrabarty, the Special Guest Editor for this issue, for his useful advice, helpful guidance, and above all for handling our numerous inquiries in a very congenial and friendly manner.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published as part of the special collection of articles "Festschrift in honour of Prof. Ramon Carbó-Dorca".
Rights and permissions
About this article
Cite this article
Malli, G.L., DiLabio, G.A., Loveland, W. et al. Dramatic relativistic and magnetic Breit effects for the superheavy reaction Og + 3Ts2 → OgTs6: prediction of atomization energy and the existence of the superheavy octahedral oganesson hexatennesside OgTs6. Theor Chem Acc 140, 137 (2021). https://doi.org/10.1007/s00214-021-02832-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02832-y