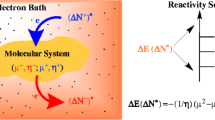
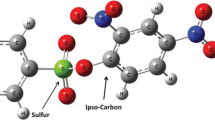
Abstract
The range of applicability of intrinsic (i.e., electronic) relative indices for quantifying electrophilicity and nucleophilicity responses (Chamorro et al. in J Phys Chem A 117(12):2636–2643, 2013) is extended to the characterization of coupling reactions of indoles with benzhydrylium ions and with the strongly electron-deficient heteroarene 4,6-dinitrobenzofuroxan. The reactivity categorization based on experimental kinetic evidence for such a electrophile/nucleophile coupling (Lakhdar et al. in J Org Chem 71(24):9088–9095, 2006) is rationalized in terms of purely electronic descriptors, revealing the polar nucleophilic activation of indole as a key factor associated with the initial rate-determining C–C coupling process.











Similar content being viewed by others
References
Mayr H, Patz M (1994) Scales of nucleophilicity and electrophilicity—a system for ordering polar organic and organometallic reactions. Angew Chem Int Ed Engl 33(9):938–957. doi:10.1002/anie.199409381
Mayr H, Bug T, Gotta MF, Hering N, Irrgang B, Janker B, Kempf B, Loos R, Ofial AR, Remennikov G, Schimmel H (2001) Reference scales for the characterization of cationic electrophiles and neutral nucleophiles. J Am Chem Soc 123(39):9500–9512. doi:10.1021/ja010890y
Chamorro E, Duque-Norena M, Perez P (2009) A comparison between theoretical and experimental models of electrophilicity and nucleophilicity. J Mol Struct Theochem 896(1–3):73–79. doi:10.1016/j.theochem.2008.11.009
Chamorro E, Duque-Norena M, Notario R, Perez P (2013) Intrinsic relative scales of electrophilicity and nucleophilicity. J Phys Chem A 117(12):2636–2643. doi:10.1021/jp312143t
Parr RG, Von Szentpaly L, Liu SB (1999) Electrophilicity index. J Am Chem Soc 121(9):1922–1924. doi:10.1021/ja983494x
Chattaraj PK, Sarkar U, Roy DR (2006) Electrophilicity index. Chem Rev 106(6):2065–2091. doi:10.1021/cr040109f
Chattaraj PK, Maiti B, Sarkar U (2003) Philicity: a unified treatment of chemical reactivity and selectivity. J Phys Chem A 107(25):4973–4975. doi:10.1021/jp034707u
Chattaraj PK, Roy DR (2007) Update 1 of: electrophilicity index. Chem Rev 107(9):PR46–PR74. doi:10.1021/cr078014b
Chattaraj PK, Giri S, Duley S (2011) Update 2 of: electrophilicity index. Chem Rev 111(2):PR43–PR75. doi:10.1021/cr100149p
Chamorro E, Duque-Norena M, Perez P (2009) Further relationships between theoretical and experimental models of electrophilicity and nucleophilicity. J Mol Struct Theochem 901(1–3):145–152. doi:10.1016/j.theochem.2009.01.014
Domingo LR, Perez P (2011) The nucleophilicity N index in organic chemistry. Org Biomol Chem 9(20):7168–7175. doi:10.1039/c1ob05856h
Perez P, Toro-Labbe A, Aizman A, Contreras R (2002) Comparison between experimental and theoretical scales of electrophilicity in benzhydryl cations. J Org Chem 67(14):4747–4752. doi:10.1021/jo020255q
Chamorro E, Chattaraj PK, Fuentealba P (2003) Variation of the electrophilicity index along the reaction path. J Phys Chem A 107(36):7068–7072. doi:10.1021/jp035435y
Ayers PW, Anderson JSM, Bartolotti LJ (2005) Perturbative perspectives on the chemical reaction prediction problem. Int J Quantum Chem 101(5):520–534. doi:10.1002/qua.20307
Ayers PW, Parr RG (2000) Variational principles for describing chemical reactions: the Fukui function and chemical hardness revisited. J Am Chem Soc 122(9):2010–2018. doi:10.1021/ja9924039
Gazquez JL, Cedillo A, Vela A (2007) Electrodonating and electroaccepting powers. J Phys Chem A 111(10):1966–1970. doi:10.1021/jp065459f
Mayr H, Ofial AR (2008) Do general nucleophilicity scales exist? J Phys Org Chem 21(7–8):584–595. doi:10.1002/poc.1325
Minegishi S, Kobayashi S, Mayr H (2004) Solvent nucleophilicity. J Am Chem Soc 126(16):5174–5181. doi:10.1021/ja031828z
Minegishi S, Mayr H (2003) How constant are Ritchie’s “constant selectivity relationships”? A general reactivity scale for n-, pi-, and sigma-nucleophiles. J Am Chem Soc 125(1):286–295. doi:10.1021/ja021010y
Bentley TW (2010) How does the s(e plus n) equation work? Comparisons with a modified Swain–Scott equation (e plus sn) and revision of the n − 1 scale of solvent nucleophilicity. J Phys Org Chem 23(1):30–36. doi:10.1002/poc.1578
Bentley TW (2010) A design to prevent floating within the n scale of nucleophilicity. J Phys Org Chem 23(9):836–844. doi:10.1002/poc.1670
Bentley TW (2011) Nucleophilicity parameters for strong nucleophiles in dimethyl sulfoxide. A direct alternative to the s(e plus n) equation. J Phys Org Chem 24(4):282–291. doi:10.1002/poc.1747
Mayr H (2011) Reply to t. W. Bentley: limitations of the s(e + n) and related equations. Angew Chem Int Ed 50(16):3612–3618. doi:10.1002/anie.201007923
Mayr H, Lakhdar S, Maji B, Ofial AR (2012) A quantitative approach to nucleophilic organocatalysis. Beilstein J Org Chem 8:1458–1478. doi:10.3762/bjoc.8.166
Mayr H, Ofial AR (2005) Kinetics of electrophile–nucleophile combinations: a general approach to polar organic reactivity. Pure Appl Chem 77(11):1807–1821. doi:10.1351/pac200577111807
Mayr H, Ofial AR (2015) A quantitative approach to polar organic reactivity. SAR QSAR Environ Res 26(7–9):619–646. doi:10.1080/1062936x.2015.1078409
Mayr H (2015) Reactivity scales for quantifying polar organic reactivity: the benzhydrylium methodology. Tetrahedron 71(32):5095–5111. doi:10.1016/j.tet.2015.05.055
Richter D, Hampel N, Singer T, Ofial AR, Mayr H (2009) Synthesis and characterization of novel quinone methides: reference electrophiles for the construction of nucleophilicity scales. Eur J Org Chem 19:3203–3211. doi:10.1002/ejoc.200900299
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103(5):1793–1873. doi:10.1021/cr990029p
Geerlings P, De Proft F (2008) Conceptual dft: the chemical relevance of higher response functions. Phys Chem Chem Phys 10(21):3028–3042. doi:10.1039/b717671f
Perez P, Chamorro E, Ayers PW (2008) Universal mathematical identities in density functional theory: results from three different spin-resolved representations. J Chem Phys 128(20):204108. doi:10.1063/1.2916714
Gazquez JL, Vela A, Galvan M (1987) Fukui function, electronegativity and hardness in the Kohn–Sham theory. Struct Bond 66:79–97
Galvan M, Vela A, Gazquez JL (1988) Chemical-reactivity in spin-polarized density functional theory. J Phys Chem 92(22):6470–6474. doi:10.1021/j100333a056
Parr RG, Chattaraj PK (1991) Principle of maximum hardness. J Am Chem Soc 113(5):1854–1855. doi:10.1021/ja00005a072
Brotzel F, Chu YC, Mayr H (2007) Nucleophilicities of primary and secondary amines in water. J Org Chem 72(10):3679–3688. doi:10.1021/jo062586z
Chamorro E, Melin J (2015) On the intrinsic reactivity index for electrophilicity/nucleophilicity responses. J Mol Model 21(3):1–3. doi:10.1007/s00894-015-2608-2
Lakhdar S, Westermaier M, Terrier F, Goumont R, Boubaker T, Ofial AR, Mayr H (2006) Nucleophilic reactivities of indoles. J Org Chem 71(24):9088–9095. doi:10.1021/jo0614339
Cacchi S, Fabrizi G (2005) Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem Rev 105(7):2873–2920. doi:10.1021/cr040639b
Humphrey GR, Kuethe JT (2006) Practical methodologies for the synthesis of indoles. Chem Rev 106(7):2875–2911. doi:10.1021/cr0505270
Gribble GW (2000) Recent developments in indole ring synthesis-methodology and applications. J Chem Soc Perkin Trans 1(7):1045–1075. doi:10.1039/a909834h
Bandini M, Eichholzer A (2009) Catalytic functionalization of indoles in a new dimension. Angew Chem Int Ed 48(51):9608–9644. doi:10.1002/anie.200901843
Nicolaou KC, Bulger PG, Sarlah D (2005) Palladium-catalyzed cross-coupling reactions in total synthesis. Angew Chem Int Ed 44(29):4442–4489. doi:10.1002/anie.200500368
Steinmetz KA, Potter JD (1996) Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc 96(10):1027–1039. doi:10.1016/s0002-8223(96)00273-8
Kochanowska-Karamyan AJ, Hamann MT (2010) Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem Rev 110(8):4489–4497. doi:10.1021/cr900211p
Sidhu JS, Singla R, Mayank JV (2016) Indole derivatives as anticancer agents for breast cancer therapy: a review. Anti-Cancer Agents Med Chem 16(2):160–173. doi:10.2174/1871520615666150520144217
Pan Q, Mustafa NR, Tang K, Choi YH, Verpoorte R (2016) Monoterpenoid indole alkaloids biosynthesis and its regulation in Catharanthus roseus: a literature review from genes to metabolites. Phytochem Rev 15(2):221–250. doi:10.1007/s11101-015-9406-4
Zhang M-Z, Chen Q, Yang G-F (2015) A review on recent developments of indole-containing antiviral agents. Eur J Med Chem 89:421–441. doi:10.1016/j.ejmech.2014.10.065
Sandtorv AH (2015) Transition metal-catalyzed c–h activation of indoles. Adv Synth Catal 357(11):2403–2435. doi:10.1002/adsc.201500374
Netz N, Opatz T (2015) Marine indole alkaloids. Mar Drugs 13(8):4814–4914. doi:10.3390/md13084814
Makosza M, Wojciechowski K (2015) Application of nucleophilic substitution of hydrogen in nitroarenes to the chemistry of indoles. Chem Heterocycl Compd 51(3):210–222. doi:10.1007/s10593-015-1687-4
Kim J, Park W (2015) Indole: a signaling molecule or a mere metabolic byproduct that alters bacterial physiology at a high concentration? J Microbiol 53(7):421–428. doi:10.1007/s12275-015-5273-3
Gupta N, Goyal D (2015) Synthesis of indole and its derivatives in water. Chem Heterocycl Compd 51(1):4–16. doi:10.1007/s10593-015-1651-3
Arora PK, Sharma A, Bae H (2015) Microbial degradation of indole and its derivatives. J Chem. doi:10.1155/2015/129159
Walsh CT (2014) Biological matching of chemical reactivity: pairing indole nucleophilicity with electrophilic isoprenoids. ACS Chem Biol 9(12):2718–2728. doi:10.1021/cb500695k
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision C01
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105(8):2999–3093. doi:10.1021/cr9904009
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. The international series of monographs on chemistry. Oxford University Press, Oxford
Jaramillo P, Domingo LR, Chamorro E, Perez P (2008) A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J Mol Struct Theochem 865(1–3):68–72. doi:10.1016/j.theochem.2008.06.022
Terrier F, Lakhdar S, Boubaker T, Goumont R (2005) Ranking the reactivity of superelectrophilic heteroaromatics on the electrophilicity scale. J Org Chem 70(16):6242–6253. doi:10.1021/jo0505526
Terrier F, Lakhdar S, Goumont R, Boubaker T, Buncel E (2004) Electrophilicity parameters for sigma-complexation by uncharged electron-deficient aromatic and heteroaromatic structures. Chem Commun 22:2586–2587. doi:10.1039/b410346d
Terrier F, Pouet M-J, Halle J-C, Hunt S, Jones JR, Buncel E (1993) Electrophilic heteroaromatic substitutions: reactions of 5-x-substituted indoles with 4,6-dinitrobenzofuroxan. J Chem Soci Perkin Trans 2(9):1665–1672. doi:10.1039/P29930001665
Perez P, Domingo LR, Aurell AJ, Contreras R (2003) Quantitative characterization of the global electrophilicity pattern of some reagents involved in 1,3-dipolar cycloaddition reactions. Tetrahedron 59(17):3117–3125. doi:10.1016/s0040-4020(03)00374-0
Domingo LR, Aurell MJ, Perez P, Contreras R (2002) Quantitative characterization of the local electrophilicity of organic molecules. Understanding the regioselectivity on diels-alder reactions. J Phys Chem A 106(29):6871–6875. doi:10.1021/jp020715j
Domingo LR, Aurell MJ, Perez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in diels-alder reactions. Tetrahedron 58(22):4417–4423. doi:10.1016/s0040-4020(02)00410-6
Jennings P, Jones AC, Mount AR, Thomson AD (1997) Electrooxidation of 5-substituted indoles. J Chem Soc Faraday Trans 93(21):3791–3797. doi:10.1039/a703128i
Acknowledgments
This work is dedicated to Professor Alberto Vela on the occasion of his 65th birthday. The continuous support from FONDECYT (Chile) through Projects 1140343(E.C.), 1140341(P.P.), and 11130589 (M.D.-N.) is gratefully acknowledged. We also are indebted to the Ministerio de Economía y Competitividad (Spain) Project CTQ2013-45646-P (L.R.D.) and the predoctoral contract from the European Social Fund BES-2014-068258 (M.R.-G.). Prof L.R.D. thanks FONDECYT for the continuous support received through the Cooperación Internacional programs. Finally, E.C. and P.P. thank the Millennium Nucleus Chemical Processes and Catalysis (CPC), Grant Number NC 120082, and the Universidad Andres Bello (UNAB) for support through research Grants DI-806-15/R and DI-793-15/R, respectively. We acknowledged the anonymous reviewers whose constructive criticism has helped us to improve the quality of our presentation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles “Festschrift in honour of A. Vela”.
Rights and permissions
About this article
Cite this article
Chamorro, E., Duque-Noreña, M., Ríos-Gutiérrez, M. et al. Intrinsic relative nucleophilicity of indoles. Theor Chem Acc 135, 202 (2016). https://doi.org/10.1007/s00214-016-1974-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1974-x