Abstract
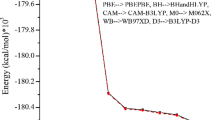
In recent years, diagnosis of diseases worldwide has been of much interest to the scientific community. Among these diagnosis methods, fluorescence spectroscopy has shown promise. Naphthoquinone and their halogenated derivatives have fluorescent properties and the presence of such substituents promote changes in the spectroscopic properties of the compounds. These properties can be studied by time dependent density functional theory methods. Relativistic effects such as spin–orbit coupling, the Hamiltonian relativistic and the basis set including relativistic corrections are essential for the accurate calculation of spectroscopic properties. For the selection of which of these factors are important for the halogenated derivatives naphthoquinone (F, Cl, Br and I) were employed in a factorial design of the 33 Type, known as a Box–Benhken design. It was observed that the DKH2 Hamiltonian and the basis set TVZ_DKH were significant for studying spectroscopic properties of these compounds. Using these parameters, the ESIPT process was investigated for halogenated compounds of naphthoquinone. It was observed that compounds containing Cl, Br and I do not have the ESIPT process, while a compound containing F showed the process having energy values, 4.69 eV for absorption energy, −1.58 eV for the proton transfer energy and 1.87 eV for the emission energy. We believe that the current study can assist in understanding the ESIPT behavior of ANQ derivatives and why the relativistic effects affect this process.


Similar content being viewed by others
References
Zhao J, Ji S, Chen Y, et al (2012) Excited state intramolecular proton transfer (ESIPT): from principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys Chem Chem Phys 14:8803–8817. doi:10.1039/c2cp23144a
Zhao G, Northrop BH, Han K, Stang PJ (2010) The effect of intermolecular hydrogen bonding on the fluorescence of a bimetallic platinum complex. J Phys Chem A 114:9007–9013. doi:10.1021/jp105009t
Levine BG, Martínez TJ (2007) Isomerization through conical intersections. Annu Rev Phys Chem 58:613–634. doi:10.1146/annurev.physchem.57.032905.104612
Park S, Kwon JE, Kim SH, et al (2009) A white-light-emitting molecule : frustrated energy transfer between constituent emitting centers. J Am Chem Soc 131:14043–14049. doi:10.1021/ja902533f
Dugave C, Demange L (2003) Cis− trans isomerization of organic molecules and biomolecules : implications and applications †. Chem Rev 103:2475–2532. doi:10.1021/cr0104375
Laurieri N, Egleton JE, Varney A et al (2013) A novel color change mechanism for breast cancer biomarker detection: naphthoquinones as specific ligands of human arylamine n-acetyltransferase 1. Plos One. doi:10.1371/journal.pone.0070600
Ferreira VF, Jorqueira A, Souza AMT et al (2006) Trypanocidal agents with low cytotoxicity to mammalian cell line: a comparison of the theoretical and biological features of lapachone derivatives. Bioorganic Med Chem 14:5459–5466. doi:10.1016/j.bmc.2006.04.046
Ramalho TC, Rocha EP (2016) Probing the ESIPT process in 2-amino-1,4-naphthoquinone: Thermodynamics properties, solvent effect and chemometric analysis. Theor Chem Acc 135:39. doi:10.1007/s00214-015-1786-4
Luo Y, Li Y, Qiu KM et al (2011) Metronidazole acid acyl sulfonamide: A novel class of anticancer agents and potential EGFR tyrosine kinase inhibitors. Bioorganic Med Chem 19:6069–6076. doi:10.1016/j.bmc.2011.08.038
Bermejo-Bescós P, Martín-Aragón S, Jiménez-Aliaga KL et al (2010) In vitro antiamyloidogenic properties of 1,4-naphthoquinones. Biochem Biophys Res Commun 400:169–174. doi:10.1016/j.bbrc.2010.08.038
Guzow K, Milewska M, Czaplewski C, Wiczk W (2010) A DFT/TD DFT study of the structure and spectroscopic properties of 5-methyl-2-(8-quinolinyl)benzoxazole and its complexes with Zn(II) ion. Spectrochim Acta - Part A Mol Biomol Spectrosc 75:773–781. doi:10.1016/j.saa.2009.11.053
Tucker SC, Honn KV (2013) Emerging targets in lipid-based therapy. Biochem Pharmacol 85:676–688. doi:10.1016/j.bcp.2012.11.028
Yang D, Zhao F, Zheng R et al (2015) A detailed theoretical investigation on the excited-state intramolecular proton-transfer mechanism of 3-BTHPB chemosensor. Theor Chem Acc 134:62. doi:10.1007/s00214-015-1664-0
López-de-Luzuriaga JM, Manso E, Monge M, Sampedro D (2015) Dual fluorescence of 4-(dimethylamino)-pyridine: a comparative linear response TDDFT versus state-specific CASSCF study including solvent with the PCM model. Theor Chem Acc 134:55. doi:10.1007/s00214-015-1659-x
Jana S, Dalapati S, Ghosh S, Guchhait N (2013) Excited state intramolecular charge transfer process in 5-(4-dimethylamino-phenyl)-penta-2,4-dienoic acid ethyl ester and effect of acceptor functional groups. J Photochem Photobiol A Chem 261:31–40. doi:10.1016/j.jphotochem.2013.04.005
Rocha MV, Carvalho HW, Lacerda LC et al (2014) Ionic desorption in PMMA-gamma-Fe2O3 hybrid materials induced by fast electrons: an experimental and theoretical investigation. Spectrochim Acta A Mol Biomol Spectrosc 117:276–283. doi:10.1016/j.saa.2013.08.029
Mancini DT, Sen K, Barbatti M et al (2015) Excited-state proton transfer can tune the color of protein fluorescent markers. ChemPhysChem 16:3444–3449. doi:10.1002/cphc.201500744
Brejc K, Sixma TK, Kitts PA et al (1997) Structural basis for dual excitation and photoisomerization of the Aequorea victoria green fluorescent protein. Proc Natl Acad Sci 94:2306–2311. doi:10.1073/pnas.94.6.2306
Gorin DJ, Toste FD (2007) Relativistic effects in homogeneous gold catalysis. Nature 446:395–403. doi:10.1038/nature05592
Philipsen P, van Lenthe E, Snijders J, Baerends E (1997) Relativistic calculations on the adsorption of CO on the (111) surfaces of Ni, Pd, and Pt within the zeroth-order regular approximation. Phys Rev B 56:13556–13562. doi:10.1103/PhysRevB.56.13556
Hemmilä I, Laitala V (2005) Progress in lanthanides as luminescent probes. J Fluoresc 15:529–542. doi:10.1007/s10895-005-2826-6
Dyall KG, van Lenthe E (1999) Relativistic regular approximations revisited: an infinite-order relativistic approximation. J Chem Phys 111:1366. doi:10.1063/1.479395
Kutzelnigg W (1997) Relativistic one-electron Hamiltonians “for electrons only” and the variational treatment of the Dirac equation. Chem Phys 225:203–222. doi:10.1016/S0301-0104(97)00240-1
Pyykkö P (2012) Relativistic effects in chemistry: more common than you thought. Annu Rev Phys Chem 63:45–64. doi:10.1146/annurev-physchem-032511-143755
Wolf A, Reiher M, Hess BA (2002) The generalized Douglas–Kroll transformation. J Chem Phys 117:9215. doi:10.1063/1.1515314
Cheng L, Stopkowicz S, Gauss J (2014) Analytic energy derivatives in relativistic quantum chemistry. Int J Quantum Chem 114:1108–1127. doi:10.1002/qua.24636
Wolff SK, Ziegler T, van Lenthe E, Baerends EJ (1999) Density functional calculations of nuclear magnetic shieldings using the zeroth-order regular approximation (ZORA) for relativistic effects: ZORA nuclear magnetic resonance. J Chem Phys 110:7689. doi:10.1063/1.478680
Green TFG, Yates JR (2014) Relativistic nuclear magnetic resonance J-coupling with ultrasoft pseudopotentials and the zeroth-order regular approximation. J Chem Phys 140:234106. doi:10.1063/1.4882678
Reiher M, Wolf A (2004) Exact decoupling of the Dirac Hamiltonian. II. The generalized Douglas–Kroll–Hess transformation up to arbitrary order. J Chem Phys 121:10945. doi:10.1063/1.1818681
Christiansen PA, Ermler WC, Pitzer KS (1985) Relativistic Effects in Chemical Systems. Annu Rev Phys Chem 36:407–432. doi:10.1146/annurev.pc.36.100185.002203
Bühl M, Reimann C, Pantazis DA et al (2008) Geometries of third-row transition-metal complexes from density-functional theory. J Chem Theory Comput 4:1449–1459. doi:10.1021/ct800172j
Pantazis DA, Chen X, Landis CR, Neese F (2008) All-electron scalar relativistic basis sets for third-row transition metal atoms. J Chem Theory Comput 4:908–919. doi:10.1021/ct800047t
Kubica A, Kowalewski J, Kruk D, Odelius M (2013) Zero-field splitting in nickel(II) complexes: a comparison of DFT and multi-configurational wavefunction calculations. J Chem Phys 138:064304. doi:10.1063/1.4790167
Arumugam K, Becker U (2014) Computational redox potential predictions: applications to inorganic and organic aqueous complexes, and complexes adsorbed to mineral surfaces. Minerals 4:345–387. doi:10.3390/min4020345
Elkechai A, Kias F, Talbi F, Boucekkine A (2014) Redox properties of biscyclopentadienyl uranium(V) imido-halide complexes: a relativistic DFT study. J Mol Model 20:2294. doi:10.1007/s00894-014-2294-5
Kühn M, Weigend F (2014) Phosphorescence lifetimes of organic light-emitting diodes from two-component time-dependent density functional theory. J Chem Phys 141:224302. doi:10.1063/1.4902013
Bonatsou S, Benítez A, Rodríguez-Gómez F et al (2015) Selection of yeasts with multifunctional features for application as starters in natural black table olive processing. Food Microbiol 46:66–73. doi:10.1016/j.fm.2014.07.011
de Azevedo ALMS, Neto BB, Scarminio IS et al (1996) A chemometric analysis of ab initio vibrational frequencies and infrared intensities of methyl fluoride. J Comput Chem 17:167–177. doi:10.1002/(SICI)1096-987X(19960130)17:2<167:AID-JCC4>3.0.CO;2-U
Ribeiro RLV, Grespan CB, Collins CH et al (1999) Optimization through Factorial Planning of the Use of Ethanol: Water as a Mobile Phase for Reversed Phase HPLC. J High Resolut Chromatogr 22:52–54. doi:10.1002/(SICI)1521-4168(19990101)22:1<52:AID-JHRC52>3.0.CO;2-T
Ferreira SLC, Bruns RE, Ferreira HS et al (2007) Box–Behnken design: An alternative for the optimization of analytical methods. Anal Chim Acta 597:179–186. doi:10.1016/j.aca.2007.07.011
Neese F (2012) The ORCA program system. Wiley Interdiscip Rev Comput Mol Sci 2:73–78. doi:10.1002/wcms.81
Pyykkö P (2004) Theoretical chemistry of gold. Angew Chem Int Ed Engl 43:4412–4456. doi:10.1002/anie.200300624
Pushpam S, Kottaisamy M, Ramakrishnan V (2013) Dynamic quenching study of 2-amino-3-bromo-1,4-naphthoquinone by titanium dioxide nano particles in solution (methanol). Spectrochim Acta - Part A Mol Biomol Spectrosc 114:272–276. doi:10.1016/j.saa.2013.05.038
Pal S, Jadhav M, Weyhermüller T et al (2013) Molecular structures and antiproliferative activity of side-chain saturated and homologated analogs of 2-chloro-3-(n-alkylamino)-1,4-napthoquinone. J Mol Struct 1049:355–361. doi:10.1016/j.molstruc.2013.06.062
Roemelt M, Beckwith MA, Duboc C et al (2012) Manganese K-edge X-ray absorption spectroscopy as a probe of the metal-ligand interactions in coordination compounds. Inorg Chem 51:680–687. doi:10.1021/ic202229b
Scherzer-Attali R, Farfara D, Cooper I et al (2012) Naphthoquinone-tryptophan reduces neurotoxic Aβ*56 levels and improves cognition in Alzheimer’s disease animal model. Neurobiol Dis 46:663–672. doi:10.1016/j.nbd.2012.03.005
Haiduke RLA, Comar M, da Silva ABF (2006) The employment of relativistic adapted Gaussian basis sets in Douglas–Kroll–Hess scalar calculations with diatomic molecules. Chem Phys 331:173–177. doi:10.1016/j.chemphys.2006.10.009
Aslan N, Cebeci Y (2007) Application of Box–Behnken design and response surface methodology for modeling of some Turkish coals. Fuel 86:90–97. doi:10.1016/j.fuel.2006.06.010
Ferreira SLC, Bruns RE, da Silva EGP et al (2007) Statistical designs and response surface techniques for the optimization of chromatographic systems. J Chromatogr A 1158:2–14. doi:10.1016/j.chroma.2007.03.051
Owens EA, Hyun H, Tawney JG, et al. (2015) Correlating Molecular Character of NIR Imaging Agents with Tissue-Specific Uptake. J Med Chem 58:4348–4356. doi:10.1021/acs.jmedchem.5b00475
Tiang JM, Butcher NJ, Minchin RF (2010) Small molecule inhibition of arylamine N-acetyltransferase Type I inhibits proliferation and invasiveness of MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun 393:95–100. doi:10.1016/j.bbrc.2010.01.087
Di Rosso ME, Barreiro Arcos ML, Elingold I et al (2013) Novel o-naphthoquinones induce apoptosis of EL-4 T lymphoma cells through the increase of reactive oxygen species. Toxicol Vitr 27:2014–2094. doi:10.1016/j.tiv.2013.08.002
Bezerra MA, Santelli RE, Oliveira EP et al (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977. doi:10.1016/j.talanta.2008.05.019
Gourlaouen C, Eng J, Otsuka M et al (2015) Quantum chemical interpretation of ultrafast luminescence decay and intersystem crossings in rhenium(I) carbonyl bipyridine complexes. J Chem Theory Comput 11:99–110. doi:10.1021/ct500846n
Acknowledgments
The authors thank the Brazilian agencies FAPEMIG, CAPES, and CNPq for the financial support of this research and UFLA for infrastructure and encouragement in this work. T.C.R. thanks also the invited professor position at the Czech Republic Center for Basic and Applied research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles “CHITEL 2015 - Torino - Italy”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
da Rocha, E.P., Castro, A.A., Ramalho, T.C. et al. Insights into the value of statistical models and relativistic effects for the investigation of halogenated derivatives of fluorescent probes. Theor Chem Acc 135, 135 (2016). https://doi.org/10.1007/s00214-016-1862-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1862-4