Abstract
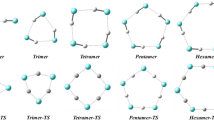
The interaction between hexafluorobenzene, \({\text {C}}_{6}{\text {F}}_{6}\), and \({\text {H}}_{2}\)O is investigated to construct a force field for molecular dynamics simulations. In order to construct the \({\text {C}}_{6}{\text {F}}_{6}\)–\({\text {H}}_{2}\)O intermolecular interaction function, the nonpermanent charge contributions, grouped in the so-called nonelectrostatic term and described using an improved Lennard-Jones model, are combined with the electrostatic energy calculated in agreement with the permanent electric quadrupole and dipole moments of \({\text {C}}_{6}{\text {F}}_{6}\) and \({\text {H}}_{2}\)O, respectively. Moreover, to test the potential energy function, BSSE-corrected energies at CCSD(T)/aug-cc-pVTZ level are calculated for three different approaches of \({\text {H}}_{2}{\text {O}}\)–\({\text {C}}_{6}{\text {F}}_{6}\). By using the constructed force field, the structure and energetics of some small aggregates [\({\text {C}}_{6}{\text {F}}_{6}\)–(\({\text {H}}_{2}{\text {O}})_{n}\) (\(n= 1{\text {--}}6\))], the formation of the first solvation shell [\({\text {C}}_{6}{\text {F}}_{6}\)–(\({\text {H}}_{2}{\text {O}})_{n}\) (\(n = 9{\text {--}}36\))] and the solvation of \({\text {C}}_{6}{\text {F}}_{6}\) by 400 molecules of \({\text {H}}_{2}\)O have been investigated. The \({\text {C}}_{6}{\text {F}}_{6}\)–(\({\text {H}}_{2}{\text {O}})_{n}\) (\(n= 1{\text {--}}6\)) small aggregates and the formation of the first solvation shell have been simulated using a microcanonical (NVE) ensemble of particles, while an isobaric–isothermal ensemble (NpT) has been used to investigate the solvation of \({\text {C}}_{6}{\text {F}}_{6}\). Moreover, in order to approximate the system formed by one \({\text {C}}_{6}{\text {F}}_{6}\) and 400 \({\text {H}}_{2}\)O molecules to a large (infinite) system, periodic boundary conditions have been imposed in the simulation of the solvation of \({\text {C}}_{6}{\text {F}}_{6}\).








Similar content being viewed by others
References
Tsuzuki S, Fujii A (2008) Nature and physical origin of CH/\(\pi\) interaction: significant difference from conventional hydrogen bonds. Phys Chem Chem Phys 10:2584–2594
Buckingham AD, Fowler P, Hutson JM (1988) Theoretical studies of van der Waals molecules and intermolecular forces. Chem Rev 88:963–988
Chalasinski G, Szczesniak MM (2000) State of the ab Initio theory of intermolecular interactions. Chem Rev 100:4227–4252
Meyer EA, Castellano RK, Diederich F (2003) An efficient algorithm for the density-functional theory treatment of dispersion interactions. Angew Chem Int Ed 42:1210–1250
Müller-Dethfels K, Hobza P (2000) Noncovalent interactions: a challenge for experiment and theory. Chem Rev 100:143–168
Kim KS, Tarakeshwar P, Lee JY (2000) Molecular clusters of \(\pi\)-systems: theoretical studies of structures, spectra, and origin of interaction energies. Chem Rev 100:4145–4185
Zhao Y, Truhlar DG (2007) Density functionals for noncovalent interaction energies of biological importance. J Chem Theory Comput 3:289–300
Riley KE, Pitoňák M, Jurečka P, Hobza P (2010) Stabilization and structure calculations for Noncovalent interactions in extended molecular systems based on wave function and density functional theories. Chem Rev 110:5023–5063
Hobza P (2012) Calculations on noncovalent interactions and databases of benchmark interaction energies. Acc Chem Res 45:663–672
Maitland GC, Rigby M, Smith EB, Wakeham WA (1987) Intermolecular forces. Clarendon Press, Oxford
Stone AJ (1996) The theory of internuclear forces. Clarendon Press, Oxford
Garau C, Frontera A, Quiñonero D, Ballester P, Costa A, Deyà PM (2004) Cation-\(\pi\) versus anion-\(\pi\) interactions: energetic, charge transfer, and aromatic aspects. J Phys Chem A 108:9423–9427
Hirshfelder JO, Curtiss CF, Bird RB (1964) Molecular theory of gases and liquids. Wiley, New York
Battaglia MR, Buckingham AD, Williams JH (1981) The Electric Quadrupole Moments of Benzene and Hexafluorobenzene. Chem Phys Lett 78:421–423
Quiñonero D, Garau C, Frontera A, Ballester P, Costa A, Deyà PM (2002) Counterintuitive interaction of anions with benzene derivatives. Chem Phys Lett 359:486–492
Alkorta I, Rozas I, Elguero J (1997) An attractive interaction between the \(\pi\)-cloud of C\(_{6}\)F\(_{6}\) and electron-donor atoms. J Org Chem 62:4687–4691
Read WG, Campbell EJ, Henderson G (1983) The rotational spectrum and molecular structure of the benzene–hydrogen chloride complex. J Chem Phys 78:3501–3508
Baiocchi FA, Williams JH, Klemperer W (1983) Molecular beam studies of hexafluorobenzene, trifluorobenzene, and benzene complexes of hydrogen fluoride. The rotational spectrum of benzene-hydrogen fluoride. J Phys Chem 87:2079–2084
Suzuki S, Green PG, Bumgarner RF, Dasgupta S, Goddard WA III, Blake GA (1992) Benzene forms hydrogen bonds with water. Science 257:942–945
Rodham DA, Suzuki S, Suenram RD, Lovas FJ, Dasgupta S, Goddard WA III, Blake GA (1993) Hydrogen bonding in the benzene–ammonia dimer. Nature 362:735–736
Jain A, Ramanathan V, Sankararamakrishnan R (2009) Lone pair \(\dots\) \(\pi\) interactions between water oxygen and aromatic residues: quantum chemical studies based on high-resolution protein structures and model compounds. Protein Sci 18:595–605
Jeziorski B, Moszynski R, Szalewicz K (1994) Perturbation theory approach to intermolecular potential energy surfaces of van der Waals complexes. Chem Rev 94:1887–1930
Mas EM, Szalewicz K, Bukowski R, Jeziorski B (1997) Pair potential for water from symmetry-adapted perturbation theory. J Chem Phys 107:4207–4218
Bukowski R, Sadlej J, Jeziorski B, Jankowsky P, Szalewicz K, Kucharski SA, Williams HL, Rice BM (1999) Intermolecular potential of carbon dioxide dimer from symmetry-adapted perturbation theory. J Chem Phys 110:3785–3803
Misquitta AJ, Szalewicz K (2002) Intermolecular forces from asymptotically corrected density functional description of monomers. Chem Phys Lett 357:301–306
Misquitta AJ, Jeziorski B, Szalewicz K (2003) Dispersion energy from density-functional theory description of monomers. Phys Rev Lett 91:033201(1)–033201(4)
Tsuzuki S, Honda K, Uchimaru T, Mikami M, Tanabe K (2000) Origin of the attraction and directionality of the NH/\(\pi\) interaction: comparison with OH/\(\pi\) and CH/\(\pi\) interactions. J Am Chem Soc 122:11450–11458
Shibasaki K, Fujii A, Mikami N, Tsuzuki S (2006) Magnitude of the CH/\(\pi\) interaction in the gas phase: experimental and theoretical determination of the accurate interaction energy in benzene-methane. J Phys Chem A 110:4397–4404
Shibasaki K, Fujii A, Mikami N, Tsuzuki S (2007) Magnitude and nature of interactions in benzene-X (X \(=\) ethylene and acetylene) in the gas phase: significantly different CH/\(\pi\) interaction of acetylene as compared with those of ethylene and methane. J Phys Chem A 111:753–758
Fujii A, Shibasaki K, Kazama T, Itaya R, Mikami N, Tsuzuki S (2008) Experimental and theoretical determination of the accurate interaction energies in benzene–halomethane: the unique nature of the activated CH/\(\pi\) interaction of haloalkanes. Phys Chem Chem Phys 10:2836–2843
Ma J, Alfè D, Michaelides A, Wang E (2009) The water-benzene interaction: insight from electronic structure theories. J Chem Phys 130:154303(1)–154303(6)
Brutschy B (1992) Ion-molecule reactions within molecular clusters. Chem Rev 92:1567–1587
Brutschy B (2000) The structure of microsolvated benzene derivatives and the role of aromatic substituents. Chem Rev 100:3891–3920
Vaupel S, Brutschy B, Tarakeshwar P, Kim KS (2006) Characterization of weak NH-\(\pi\) intermolecular interactions of ammonia with various substituted systems. J Am Chem Soc 128:5416–5426
Pirani F, Brizi S, Roncaratti LF, Casavecchia P, Cappelletti D, Vecchiocattivi F (2008) Beyond the Lennard-Jones model: a simple and accurate potential function probed by high resolution scattering data useful for molecular dynamics simulations. Phys Chem Chem Phys 10:5489–5503
Faginas Lago N, Huarte-Larrañaga F, Albertí M (2009) On the suitability of the ILJ function to match different formulations of the electrostatic potential for water-water interactions. Eur Phys J D 55:75–85
Pirani F, Albertí M, Castro A, Moix M, Cappelletti D (2004) Atom-bond pairwise additive representation for intermolecular potential energy surfaces. Chem Phys Lett 394:37–44
Albertí M, Pirani F (2011) Features of Ar solvation shells in neutral and ionic clustering: the competitive role of two-body and many-body interactions. J Phys Chem A 115:6394–6404
Albertí M, Aguilar A, Lucas JM, Pirani F, Cappelletti D, Coletti C, Re N (2006) Atom-bond pairwise additive representation for cation-benzene potential energy surfaces: an ab initio validation study. J Phys Chem A 110:9002–9010
Albertí M, Aguilar A, Lucas JM, Pirani F, Coletti C, Re N (2009) Atom-bond pairwise additive representation for halide-benzene potential energy surfaces: an ab initio validation study. J Phys Chem A 113:14606–14614
Kitaura K, Morokuma K (1976) A new energy decomposition scheme for molecular interactions within the Hartree–Fock approximation. Int J Quantum Chem 10:325–340
Kitaura K, Morokuma K (1981) In: Politzer P, Truhlar DG (eds) Chemical applications of electrostatic potentials. Plenum Press, New York
Chen W, Gordon MS (1996) Energy decomposition analyses for many-body interaction and applications to water complexes. J Phys Chem 100:14316–14328
Albertí M, Aguilar A, Cappelletti D, Laganà A, Pirani F (2009) On the development of an effective model potential to describe water interaction in neutral and ionic clusters. Int J Mass Spectrom 280:50–56
Bukowski R, Cencek W, Jankowski P, Jeziorska M, Jeziorski B, Lotrich VF, Kucharski SA, Misquitta AJ, Moszyński R, Patkowski K, Podeszwa R, Rybak S, Szalewicz K, Williams HL, Wheatley RJ, Wormer PES, Zuchowski PS SAPT (2006) Program package; University of Delaware and University of Warsaw: Newark, Delaware and ul. Pasteura 1, 02-093 Warsaw
Albertí M, Aguilar A, Huarte-Larrañaga F, Lucas JM, Pirani F (2014) Benzene-hydrogen bond (C\(_{6}\)H\(_{6}\)-HX) interactions: the influence of the X nature on their strength and anisotropy. J Phys Chem A 118:1651–1662
Albertí M, Faginas Lago N, Pirani F (2012) Benzene-water interaction: from gaseous dimers to solvated aggregates. Chem Phys 399:232–239
Albertí M, Aguilar A, Lucas JM, Pirani F (2012) Competitive role of CH\(_{4}\)-CH\(_{4}\) and CH-\(\pi\) interactions in C\(_{6}\)H\(_{6}\)-(CH\(_{4}\))\(_{n}\) aggregates: the transition from dimer to cluster features. J Phys Chem A 116:5480–5490
Sherrill CD, Takatani T, Hohenstein EG (2009) An assessment of theoretical methods for nonbonded interactions: comparison to complete basis set limit coupled-cluster potential energy curves for the benzene dimer, the methane dimer, benzene–methane, and benzene-H\(_{2}\)S. J Phys Chem A 113:10146–10159
Tauer TP, Derrick ME, Sherrill CD (2005) Estimates of the ab initio limit for sulfur-\(\pi\) interactions: the H\(_{2}\)S-benzene dimer. J Phys Chem A 109:191–196
Crittenden DL (2009) A systematic CCSD(T) study of long-range and noncovalent interaction between benzene and a series of first- and second-row hydrides and rare gas atoms. J Phys Chem A 113:1663–1669
Bloom JWG, Raju RK, Wheeler SE (2012) Physical nature of substituent effects in XH/\(\pi\) interactions. J Chem Theory Comput 8:3167–3174
Allesch M, Schwegler E, Galli G (2007) Structures and hydrophobic hydration of benzene and hexafluorobenzene from first principles. J Phys Chem B 111:1081–1089
Head-Gordon M, Pople JA, Frisch J (1998) MP2 energy evaluation by direct methods. Chem Phys Lett 153:503–506
Head-Gordon M, Head-Gordon T (1994) Analytic MP2 frequencies without fifth-order storage. Theory and application to bifurcated hydrogen bonds in the water hexamer. Chem Phys Lett 220:122–128
Woon DE, Dunning TH Jr (1993) Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Chem Phys 98:1358–1371
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr J A, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam MJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian Inc, Wallingford CT
Purvis GD III, Bartlett A (1982) Full coupled-cluster singles and doubles model—the inclusion of disconnected triples. J Chem Phys 76:1910–1918
Scuseria GE, Janssen CL, Schaefer HF III (1988) An efficient reformulation of the closed-shell coupled cluster single and double excitation (CCSD) equations. J Chem Phys 89:7382–7387
Pople JA, Head-Gordon M, Raghavachari K (1987) Quadratic configuration interaction. A general technique for determining electron correlation energies. J Chem Phys 87:5968–5975
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Simon S, Duran M, Dannenberg JJ (1996) How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers? J Chem Phys 105:11024–11031
Gallivan JP, Dougherty DA (1999) Can lone pairs bind to a \(\pi\) system? The water\(^{\dots }\)hexafluorobenzene interaction. Org Lett 1:103–105
Amicangelo JC, Irwin DG, Lee CJ, Romano NC, Saxton NL (2013) Experimental and theoretical characterization of a lone pair-\(\pi\) complex: water–hexafluorobenzene. J Phys Chem A 117:1336–1350
Denbigh KG (1940) The polarizabilities of bonds-I. Trans Faraday Soc 36:936–948
Smith RP, Mortensen EJ (1960) Bond and molecular polarizability tensors. I. Mathematical treatment of bond tensor additivity. J Chem Phys 32:502–507
Pirani F, Cappelletti D, Liuti G (2001) Range, strength and anisotropy of intermolecular forces in atom–molecule systems: an atom–bond pairwise additive approach. Chem Phys Lett 350:286–296
Cambi R, Cappelletti D, Liuti G, Pirani F (1991) Generalized correlation in terms of polarizability for van der Waals potential parameters calculations. J Chem Phys 95:1852–1861
Albertí M, Castro A, Laganà A, Moix M, Pirani F, Cappelletti D, Liuti G (2005) A molecular dynamics investigation of rare-gas solvated cation-benzene clusters using a new model potential. J Phys Chem A 109:2906–2911
Capitelli M, Cappelletti D, Colonna G, Gorse C, Laricchiuta A, Liuti G, Longo S, Pirani F (2007) On the possibility of using model potentials for collision integral calculations of interest for planetary atmospheres. Chem Phys 338:62–68
Albertí M, Aguilar A, Lucas JM, Pirani F (2010) A generalized formulation of ion-\(\pi\) electron interactions: role of the nonelectrostatic component and probe of the potential parameter transferability. J Phys Chem A 114:11964–11970
Albertí M, Aguilar A, Bartolomei M, Cappelletti D, Laganà A, Lucas JM, Pirani F (2008) A study to improve the van der Waals component of the interaction in water clusters. Phys Script 78:058108(1)–058108(7)
Soper AK (2007) Joint structure refinement of X-ray and neutron diffraction data on disordered materials: application to liquid water. J Phys Cond Mat 19:335206(1)–335206(18)
Nosé SA (1984) Molecular dynamics method for simulations in the canonical ensemble. Mol Phys 52:255–268
Berendsen HJC, Postma JPM, Vangunsteren WF, Dinola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Ewald PP (1921) Evaluation of optical and electrostatic lattice potentials. Ann Phys 64:253–287
Wallqvist A, Teleman O (1991) Properties of flexible water models. Mol Phys 74:515–533
Albertí M, Faginas Lago N, Laganà A, Pirani F (2011) A portable intermolecular potential for molecular dynamics studies of NMA–NMA and NMA–H\(_{2}\)O aggregates. Phys Chem Chem Phys 13:8422–8432
Kirkpatrick S, Gelatt CD Jr, Vecchi MP (1983) Optimization by simulated annealing. Science 220:671–680
Humphrey W, Dalke A, Schulten K (1996) VMD-visual molecular dynamics. J Mol Graphics 14:33–38
Acknowledgments
M. Albertí, A. Aguilar, F. Huarte-Larrañaga and J. M. Lucas acknowledge financial support from the Ministerio de Educación y Ciencia (Spain, Project CTQ2013-41307-P) and the Generalitat de Catalunya (2009SGR-17). Also thanks are due to the Center de Supercomputació de Catalunya CESCA-C4 and Fundació Catalana per a la Recerca for the allocated supercomputing time. A. Amat thanks FP7-NMP-2009 Project 246124 “SANS” for financial support. F. Pirani acknowledges financial support from the Italian Ministry of University and Research (MIUR) for PRIN Contracts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albertí, M., Amat, A., Aguilar, A. et al. A molecular dynamics study of the evolution from the formation of the \({\text {C}}_{6}{\text {F}}_{6}\)–(\({\text {H}}_{2}{\text {O}})_{n}\) small aggregates to the \({\text {C}}_{6}{\text {F}}_{6}\) solvation. Theor Chem Acc 134, 61 (2015). https://doi.org/10.1007/s00214-015-1662-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-015-1662-2