Abstract
Purpose
Globally, sepsis, which is a major health issue resulting from severe infection-induced inflammation, is the fifth biggest cause of death. This research aimed to evaluate, for the first time, the molecular effects of gabapentin's possible nephroprotective potential on septic rats by cecal ligation and puncture (CLP).
Methods
Sepsis was produced by CLP in male Wistar rats. Evaluations of histopathology and renal function were conducted. MDA, SOD, GSH, TNF-α, IL-1β, and IL-6 levels were measured. qRT-PCR was utilized to determine the expression of Bax, Bcl-2, and NF-kB genes. The expression of Nrf-2 and HO-1 proteins was examined by western blotting.
Results
CLP caused acute renal damage, elevated the blood levels of creatinine, BUN, TNF-α, IL-1β, and IL-6, reduced the expression of Nrf-2 and HO-1 proteins and the Bcl-2 gene expression, and upregulated NF-kB and Bax genes. Nevertheless, gabapentin dramatically diminished the degree of the biochemical, molecular, and histopathological alterations generated by CLP. Gabapentin reduced the levels of proinflammatory mediators and MDA, improved renal content of GSH and SOD, raised the expression of Nrf-2 and HO-1 proteins and Bcl-2 gene, and reduced the renal expression of NF-kB and Bax genes.
Conclusion
Gabapentin mitigated the CLP-induced sepsis-related acute kidney injury through up-regulating Nrf-2/HO-1 pathway, repressing apoptosis, and attenuating the oxidative stress status by reducing the levels of the proinflammatory mediators and enhancing the antioxidant status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis, an uncontrolled hyperinflammatory reaction toward infection, is the primary lethal reason in intensive care units (Zarjou and Agarwal 2011). The generation of multiple organ malfunction and systemic inflammatory reactions in critically ill patients due to acute infections is marked by impairments of liver, lung, cardiovascular, renal, and gastrointestinal systems (Singer et al. 2016). One of the most frequent sepsis consequences is acute kidney injury (AKI) (Gonçalves et al. 2010). AKI is developed in about 50% of septic patients, contributing to 70% of septic patient deaths in intensive care units (Höcherl et al. 2010; Lopes et al. 2010). The cecal ligation-puncture (CLP) model closely resembles human sepsis; thus, scientists usually use it to assess the potential protective impacts of many drugs screened for their possible activity against septic shock (Brooks et al. 2014).
Despite the lack of a clear etiology, sepsis-induced AKI has been linked to tubular dysfunction caused by inflammatory responses and oxidative stress, which are aggravated by cytokines release. It is well established that the nuclear factor kappa B (NF-kB) modulates a wide range of genes participating in the innate immune reaction of the body (Fawzy et al. 2021; Zaki et al. 2022). Several inflammatory mediators, such as interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), are controlled by NF-kB (Abd El-Baky et al. 2020; Alaaeldin et al. 2022). It has been shown that in polymicrobial septic models, suppressing NF-kB maintains the balance between inflammatory and anti-inflammatory mediators (Brown and Jones 2004; Bitto et al. 2012; Alaaeldin et al. 2023).
The transcriptional factor Nuclear factor erythroid 2- related factor 2 (Nrf-2) supports the cellular defense mechanisms and protects the cells against inflammation and oxidative stress (Vomund et al. 2017). Because of oxidative stress, Nrf-2 secretes an inhibitory protein from its cytoplasm that moves to the nucleus and stimulates many genes implicated in the antioxidant defense mechanism, such as heme oxygenase-1 (HO-1) and superoxide dismutase (SOD) (Vomund et al. 2017; El-Emam et al. 2020). Deficiency of Nrf-2 promotes oxidative stress, which enhances the expression of NF-kB-mediated cytokines, whereas NF-kB is more easily activated in oxidative environments (Yerra et al. 2013).
The structural counterpart of gamma amino butyric acid (GABA), Gabapentin, was prescribed for treating epilepsy for the first time in 1994. It has been shown that Gabapentin modulates calcium influx in nerve terminals by blocking calcium channels, specifically with the α2-δ1 subunit (Maneuf et al. 2006). Thus, Gabapentin regulates several neurotransmitters release, like GABA, noradrenaline, serotonin, glutamate, and substance P (SP) (Cai et al. 2012). It was shown that Gabapentin has analgesic effects in addition to its potent effect on suppressing partial seizures (Gordh et al. 2008). Moreover, it protected rat myocardium from doxorubicin-induced injury by decreasing myocardial caspase-8 and JNK contents (Samra et al. 2021). Moreover, it reduced colitis induced by trinitro benzene sulfonic acid through altering mast cell signaling. It modulated the inflammatory genes participated in the pathogenesis of inflammatory bowel disease by inhibiting their activation and stimulating the peroxisome proliferator-activated receptor gamma (PPAR- γ) (de Brito et al. 2020).
Searching for new pharmacological potentials for natural (Sabra et al. 2022) or synthetic (Shytaj et al. 2022) agents recently attracted attention (Fawzy et al. 2023a). Consequently, for the first time, the current study investigated the possible potential nephroprotective effect of Gabapentin against AKI induced by CLP-evoked sepsis in rats by evaluating Nrf2/ HO-1, apoptosis, and oxidative stress signaling pathways.
Materials and methods
Drugs, chemicals, and reagents
By Eipico Pharmaceutical Company (Cairo, Egypt), Gabapentin and vitamin C were supplied. Prior to usage, isotonic saline solution was used to dissolve the drugs.
TRIzol reagent (Life Technologies, United Kingdom, Cat no: 15596026), Cell lysis buffer (Invitrogen, United Kingdom, Cat no: FNN0011), Phosphate-Buffered Saline (10X) pH 7.4 (PBS) (Invitrogen, United Kingdom, Cat no: AM9624), 2-Mercaptoethanol (Life Technologies, United Kingdom Cat no: 35602BID), and Tris-buffered Saline (TBS-T) (Life Technologies, United Kingdom, Cat no: 37535) were utilized.
Animals
From Minia University, College of Medicine (Minia, Egypt), 120 male Wistar rats (190 ± 10 g) were obtained. They were put in polypropylene cages for a week prior to the experiment, with unrestricted access to conventional laboratory water and food. Following regulations set out by Minia University, College of Pharmacy's Research Ethics Committee, all animal experiments and care were conducted (license number: ES 07/2021).
Induction of sepsis
As reported, the CLP was utilized as a sepsis model (Ibrahim et al. 2020). Following cleaning with a ten percent povidone-iodine solution and shaving the abdominal wall, xylazine (10 mg/kg) and ketamine (100 mg/kg) were given intraperitoneally (i.p.) to anesthetize rats (Wu et al. 2018). After that, a surgical cut was created in the lower left abdominal region. After cecal exteriorization and ligation with silk surgical suture thread (0.3-mm), two 18-gauge syringe needle punctures were made in the ligated cecal region using an 18-gauge needle. Ligation of the cecum was done at 75% of its whole length. Without CLP, identical procedures were performed on rats who experienced a sham operation.
Experimental design
Rats were allocated at random into 6 groups (each of 10 rats):
Sham group: Rats were i.p. injected with normal saline (0.5 ml) daily for four days, while the operation was carried out without CLP on day 4.
Gabapentin 100 group: Rats were i.p. injected for four days with 100 mg/kg of Gabapentin.
CLP group: Rats were i.p. injected with normal saline (0.5 ml) daily for four days, while CLP procedure was carried out on day 4.
CLP/Gabapentin 50 group: Rats were i.p. injected for four days with 50 mg/kg of Gabapentin, while CLP procedure was carried out on day 4 (Motavallian et al. 2021).
CLP/Gabapentin 100 group: Rats were i.p. injected for four days with 100 mg/kg of Gabapentin, while CLP procedure was carried out on day 4 (Motavallian et al. 2021).
CLP/vitamin C group: Rats were i.p. injected a single dose of 200 mg/kg of vitamin C on day 4 after CLP procedure (Zhang et al. 2021).
Twenty-four hours following the CLP procedure, all groups experienced animal scarification.
To conduct the survival study, the same previously indicated groups of 60 rats were randomly assigned. The survival rates of all groups were monitored for 10 days.
Samples collection and tissue preparation
On the fifth day, the rats were given an intraperitoneal injection of 1.6 g/kg of 25% urethane to induce anesthesia (Abdelzaher et al. 2021). Abdominal aortic arteries were accessed to collect the blood samples. After collecting serum for biochemical analysis by centrifugation at 4000 g for 15 min, at -20 °C, the samples were preserved. Rapid dissection and washing in ice-cold (10X) PBS, pH 7.4 was performed on the kidney tissues. Four sample portions of kidney tissue were obtained. The first part was promptly frozen in liquid nitrogen and maintained for further biochemical investigation at -20 °C. For additional western blotting analysis and quantitative real-time PCR (qRT-PCR) examination, the second and third parts were preserved at -80 °C. For histological investigations, the fourth part was finally preserved in formaldehyde (10%).
Assessment of kidney functions
Following the manufacturer's guidelines, kidney function tests were done using a blood urea nitrogen (BUN) measurement kit (Cat no: 1001331, SPINREACT, Ctra. Santa Coloma, Spain) and a serum creatinine determination kit (Cat no: 11734, Biosystems, Barcelona, Spain).
Assessment of oxidative stress status
In PBS (10 mM), renal samples were homogenized at pH of 7.4. After mixing the homogenates, they were centrifuged for 10 min at 4000 g at 4 °C. The biochemical analysis required the supernatant, which was collected.
As directed by the manufacturer, the obtained supernatant was tested for the presence of renal reduced glutathione (GSH) (Biodiagnositic, Giza, Egypt, Cat no: GR 2511), malondialdehyde (MDA) (Biodiagnositic, Giza, Egypt, Cat no: MD 2529), and superoxide dismutase (SOD) (Biodiagnositic, Giza, Egypt, Cat no: SD 2521).
Assessment of serum TNF-α, IL-1β, and IL-6 levels
Inflammatory cytokines in the blood were assessed by ELISA kits specific for rats to detect TNF-α (Elabscience, Texas, United States, Cat no: E-EL-R0019), IL-1β (Elabscience, Texas, United States, Cat no: E-EL-R0012), and IL-6 (Elabscience, Texas, United States, Cat no: E EL R0015).
Quantitative real-time polymerase chain reaction
A kidney sample weighing 100 mg was ultrasonically homogenized by Branson Digital Sonifier® ultrasonic cell homogenizer (SFX 550, Danbury, Connecticut, United States) with TRIzol reagent (1 mL). Total RNA amount was evaluated, and the purity was assessed as the ratio of A260/A280. qRT-PCR was performed on RNA samples with a purity score of 1.7 or above. Using the Revert Aid First Strand cDNA Synthesis Kit (Thermo fisher scientific, United Kingdom, Cat no: K1622), total RNA (in equal quantities) were transformed into cDNA for every sample. Using single-stranded cDNAs, real-time PCR was performed. Table 1 contains the primer sequences. Using Thermo Scientific Maxima SYBR Green qPCR Master Mix (2X) (Thermo fisher scientific, United Kingdom, Cat no: K0251) and StepOne real-time PCR Detection System (Ref no: 4369074, Applied Biosystems, Singapore) were utilized to produce PCR process. mRNA levels were evaluated by the comparative cycle threshold technique following the adjustment using GAPDH as a reference gene.
Western blotting
Homogenization of renal tissue samples was performed using cell lysis buffer (100 mM NaCl, Triton X-100 buffer (0.5%), EDTA (1 mM), and Tris (20 mM)). For five minutes, protein homogenates (30 μg) were incubated with a loading buffer containing 2-mercaptoethanol before being exposed to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE, 12%) at 100 V for two hours.
The Bio-Rad Trans-Blot SD Cell instrument (Bio-Rad, Hercules, California, United States) was used for electroblotting and electrophoresis. Then, for one hour, proteins were blotted onto PVDF membranes and blocked in TBS-T blocking solution containing Tween-20 (0.05%) and non-fat milk (5% w/v). Incubation with primary antibodies, including Anti-Nrf2 antibody (1:1000) (Abcam, Massachusetts, United States, Cat no: ab92946), Anti-HO-1 antibody (1:1000) (Massachusetts, United States, Cat no: ab52947), and β-actin (Santa Cruz Biotechnology, California, United States, Cat no: sc-47778) was performed at 4 °C overnight. In a blocking buffer solution, the secondary antibody horseradish peroxidase-conjugated polyclonal immunoglobulin (1:5000) (Cell Signaling Technologies Inc., Massachusetts, United States, Cat no: 7074) was utilized. The luminescent image analyzer (LAS-4000, Fujifilm Co., Tokyo, Japan) and Chemiluminescence kit (GE Healthcare, Little Chalfont, United Kingdom) were used to identify immunoreactive proteins following the manufacturer's recommendations. Then, The Image Analysis Java (ImageJ, 1.8.0_172) program was utilized to achieve densitometric analysis. After normalizing to the relevant β -actin levels, data were compared to the sham group.
Histopathological examination
10% neutral buffered formalin solution was used for renal tissue fixation, and the traditional hematoxylin and eosin (H&E) staining was done on kidney tissues (Bancroft and Gamble 2008). Sections were viewed and analyzed by experts in the field with a high-quality digital camera placed on the microscope (Olympus, Tokyo, Japan).
Statistical analysis
The findings were depicted as mean ± standard deviation (n = 10). ANOVA was performed, proceeded by the Tukey-Kramar post-analytic test. Graph Pad Prism 7 (Graph Pad Software, Boston, MA, United States) was utilized to conduct statistical analyses. The findings were regarded as statistically significant at p-values lower than 0.05.
Results
Gabapentin's effect on survival rate of septic rats
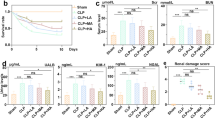
Figure 1 demonstrates that 40% of the untreated septic rats died within the first day of the operation, in contrast to the sham-operated group, which had no deaths throughout the entire period of observation (10 days). In addition, 60% of the untreated CLP group died by day two. Conversely, the septic groups that received Gabapentin or vitamin C exhibited 10% mortality on the first postoperative day. Furthermore, 40% of the rats died during the overall time of monitoring for Gabapentin treated groups and 30% for vitamin C-treated group. In comparison to the untreated septic group (20%), overall survival was significantly (p < 0.05) higher for both Gabapentin (60%) and vitamin C (70%).
Gabapentin's effect on kidney functions
Serum BUN besides creatinine concentrations were assessed to appraise the beneficial impact of Gabapentin on CLP-induced sepsis-related AKI. As regard to sham control group, the CLP group demonstrated a marked (P < 0.05) increase in serum creatinine and BUN concentration. The blood concentrations of creatinine and BUN dramatically (P < 0.05) declined in the Gabapentin-treated and vitamin C-treated groups as contrasted to the CLP group. Furthermore, as presented in Fig. 2, the serum creatinine levels of the CLP/Gabapentin 100 group declined notably (P < 0.05) compared to septic rats treated with gabapentin 50 mg.
Serum levels of BUN (A) and creatinine (B). The bars depict mean ± SD. Significant differences were analyzed using one-way ANOVA (n = 10), where a; p < 0.05, by contrast with sham control group, b; p < 0.05, by contrast with CLP group, c; p < 0.05, by contrast with CLP/Gabapentin 50 group. CLP: cecal ligation puncture, BUN: Blood urea nitrogen
Gabapentin's effect on renal SOD, GSH and MDA
Figure 3 depicts SOD, GSH, and MDA levels in the renal tissues of various groups. The renal amounts of GSH and SOD in the CLP group were markedly (P < 0.05) lower, with respect to sham control rats. However, by contrast with the CLP group, treatment with Gabapentin (the two dosages) or vitamin C (the positive control) markedly (P < 0.05) attenuated these alterations. Notably, as regards the low dose of gabapentin, the higher dose significantly (P < 0.05) escalated the GSH and SOD levels.
Renal tissue levels of SOD (A), GSH (B), and MDA (C). The bars depict mean ± SD. Significant differences were analyzed using one-way ANOVA (n = 10), where a; p < 0.05, by contrast with sham control group, b; p < 0.05, by contrast with CLP group, c; p < 0.05, by contrast with CLP/Gabapentin 50 group. CLP: cecal ligation-puncture, GSH: reduced glutathione, SOD: superoxide dismutase, MDA: malondialdehyde
Also, MDA was considerably (P < 0.05) higher in the CLP group, in comparison to sham control group. Conversely, MDA levels considerably (P < 0.05) dropped in rats treated with Gabapentin and vitamin C, in contrast to CLP group.
Gabapentin's effect on serum TNF-α, IL-6, and IL-1β levels
After CLP, TNF-α, IL-6, and IL-1β blood levels were markedly (P < 0.05) increased with respect to sham control group, as depicted in Fig. 4. Nevertheless, when rats were given Gabapentin or vitamin C treatments, their blood levels of these cytokines were markedly (P < 0.05) decreased, by contrast with CLP group. Interestingly, TNF-α level was much lower (P < 0.05) in septic rats treated with the high dose of gabapentin, by contrast with the low dose.
Serum levels of TNFα (A), IL-6 (B), and IL-1β (C). The bars depict mean ± SD. Significant differences were analyzed using one-way ANOVA (n = 10), where a; p < 0.05, by contrast with sham control group, b; p < 0.05, by contrast with CLP group, c; p < 0.05, by contrast with CLP/Gabapentin 50 group. CLP: cecal ligation-puncture, TNF-α: tumor necrosis factor alpha, IL-1β: interleukin 1 beta, IL-6: interleukin 6
Gabapentin's effect on NF-kB, Bax, and Bcl2 genes expression
CLP group demonstrated a significant (P < 0.05) elevation in mRNA level of renal NF-kB expression, by contrast with sham control group. While gabapentin treatment demonstrated considerable (P < 0.05) dose-dependent suppression of renal NF-kB gene expression, as opposed to the CLP group.
Moreover, Bax and Bcl-2 mRNA levels were analyzed to assess the gabapentin's effect on altering factors affecting apoptosis. As illustrated in Fig. 5, with respect to sham control group, CLP dramatically (P < 0.05) elevated the expression of the Bax gene and declined (P < 0.05) the expression of the Bcl-2 gene. However, as contrasted to CLP group, the expression of Bcl-2 gene was dramatically (P < 0.05) elevated and that of Bax was markedly (P < 0.05) decreased in both the Gabapentin-treated septic rats and the vitamin C-treated septic rats.
NF-kB (A), Bax (B), and Bcl-2 (C) mRNA levels in renal tissues before and after Gabapentin treatment in CLP-induced sepsis. Quantitative RT-PCR was used to analyze the gene expression of different groups. Expression was compared to the sham control group after being adjusted to that of GAPDH gene. The bars depict mean ± SD. Significant differences were analyzed using one-way ANOVA (n = 10), where a; p < 0.05, by contrast with sham control group, b; p < 0.05, by contrast with CLP group, c; p < 0.05, by contrast with CLP/Gabapentin 50 group. Bcl-2: B-cell lymphoma 2, Bax: (Bcl-2)-associated X protein, NF- kB: nuclear factor-kappa B, CLP: Cecal ligation-puncture
Gabapentin's effect on Nrf-2 and HO-1 proteins expression
As indicated in Fig. 6, after the band intensities have been adjusted to the internal control β-actin, western blot analysis showed a dramatic (P < 0.05) downregulation of renal Nrf-2 and HO-1 protein of septic untreated rats in comparison to sham control rats. Nevertheless, Gabapentin 100 administration considerably (P < 0.05) upsurged Nrf-2 protein expression by contrast with CLP animals. Additionally, the protein expression of HO-1 was considerably (P < 0.05) increased in the Gabapentin treated septic rats and vitamin C-treated septic rats, in contrast to the CLP group.
Protein expression of Nrf-2 and HO-1. (a) Representative western blots of Nrf-2, HO-1, and β-actin proteins in different groups. (b & c) Proteins expressions were determined densitometrically, using bands in (A) after the band intensities have been adjusted to the internal control β-actin, as fold change corresponding to that of sham control rats. The bars depict mean ± SD. One-way ANOVA (n = 3) was used to identify significant differences, where a; p < 0.05, by contrast with sham control group, b; p < 0.05, by contrast with CLP group. HO-1: Heme oxygenase 1, Nrf-2: nuclear factor erythroid 2-related factor 2, CLP: Cecal ligation-puncture
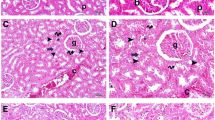
Histopathological results
As depicted in Fig. 7 and Table 2, CLP group revealed collapsed and necrosed renal glomeruli with widened Bowman’s capsule, degenerated tubules, and areas of interstitial hemorrhage in comparison to sham control group which exhibited normal glomeruli, tubules, and vasculature. Furthermore, CLP/Gabapentin 50 group revealed few necrosed renal glomeruli with widened Bowman’s capsule, tubules showing cloudy swelling, cytoplasmic vacuolations, and areas of interstitial hemorrhage. In addition, CLP/Gabapentin 100 group showed near-normal renal glomeruli, mild tubular cytoplasmic vacuolation, and normal vasculature. However, vitamin C-treated group showed minimal areas of interstitial hemorrhage, mild tubular cytoplasmic vacuolation, and degeneration.
Discussion
Sepsis is a major global challenge facing medical researchers, physicians, and emergency medicine nowadays due to its complex pathophysiology (Macdonald et al. 2017). AKI is among the highest serious consequences of sepsis, contributing to its increased number of deaths (Gómez and Kellum 2019).
The current study is the first to evaluate the possible potential nephroprotective impact of Gabapentin on CLP-evoked sepsis in rats. Due to the high mortality rate associated with sepsis, we began our investigation by analysing the Gabapentin's effect on the septic rats' survival. Our findings revealed 40% and 80% mortality from sepsis on the first and fourth days, respectively, following surgery. Surprisingly, we demonstrated that early Gabapentin administration before sepsis induction resulted in a significant reduction in mortality to 10% and 40% on the first and fourth days, respectively, following surgery.
The CLP method has been shown to cause sepsis, further renal impairment, and AKI (Yan et al. 2019b). For instance, Luo et al. have documented that resveratrol attenuated the elevated blood levels of creatinine and BUN after sepsis (Luo et al. 2020). In the current research, the concentration of these markers significantly upsurged in the CLP-evoked septic rats. However, they declined considerably in the septic rats treated with Gabapentin, showing that Gabapentin improved kidney function and reduced renal damage.
The severity of sepsis can be increased by oxidative stress and inflammation (Galley 2011; Abdelnaser et al. 2023). As indicators of early mortality in septic rats, SOD and GSH levels were reported by Ritter et al. (Ritter et al. 2003). The current investigation revealed that SOD and GSH, as antioxidant factors, were reduced during the sepsis but dramatically increased, in a dose dependent manner, following the treatment with Gabapentin resulting in promoting the antioxidant status and repressing the oxidative stress condition, suggesting the Gabapentin's renal antioxidant potential of the septic rats. Similarly, previous studies demonstrated that Gabapentin markedly reduced oxidative stress in several organs, including brain, lung, and retina (Abdel-Salam et al. 2012; Yosri et al. 2018; Ola et al. 2019).
Moreover, oxidative stress and apoptosis have been illustrated to be promoted by MDA, which is produced as a result of reactive oxygen species-induced lipid peroxidation (Requena et al. 1996; Fawzy et al. 2022). Our research indicated that the untreated septic rats had significant higher renal MDA levels than the sham group; however, Gabapentin administration significantly lowered these MDA levels, suggesting that Gabapentin may have a role in reducing oxidative stress and attenuating apoptosis against CLP-evoked sepsis.
As a crucial transcription factor, NF-kB regulates the expression of pro-inflammatory cytokines (Surh and Na 2008; Fathy et al. 2019; Abdellatef et al. 2021). Nrf-2 is an essential transcription factor, that modulates the transcription of multiple genes, including HO-1 (Yerra et al. 2013). A deficiency of Nrf-2 promotes oxidative stress, which stimulates the expression of NF-kB-mediated cytokines because NF-kB is more easily activated in an oxidative environment (Li et al. 2008; Fawzy et al. 2023b). Furthermore, it has been reported that the Nrf2 target proteins, phase II detoxifying enzymes and HO-1, control inflammation by inhibiting NF-kB (Park et al. 2015). Sepsis was demonstrated to upsurge the expression of NF-kB and repress Nrf-2 signaling pathway, which further stimulates the expression of TNF-α, IL-1β, and IL-6 (Cai et al. 2017; Wang et al. 2018). Our findings demonstrated that Gabapentin inhibited the expression of TNF-α, IL-1β, and IL-6 by suppressing NF-kB gene expression and activating Nrf-2/HO-1 signaling pathway.
The Bcl-2 family is crucial in cells' internal signaling of apoptosis. The Bcl-2 family members, the pro- and anti-apoptotic genes (Bax and Bcl-2, respectively), have a direct contribution to apoptosis, which is affected by their balance (Chittenden et al. 1995). It has been demonstrated that NF-kB upregulation increased the expression of Bax, the cell death protein, and decreased the expression of the Bcl-2, inducing apoptosis (Zhang et al. 2017; Alaaeldin et al. 2020, 2021). Consequently, the increased Bax/Bcl-2 ratio dramatically promotes apoptosis by changing the structure of the mitochondria and its potential and promoting the apoptotic signalling cascade (Wang et al. 2016). For instance, Lin et al. have demonstrated that fish oil reduced the renal apoptosis induced by CLP by decreasing the expression of Bax/Bcl-2 ratio (Lin et al. 2019).
In accordance with others, our results showed that, in untreated septic rats, the expression of the Bax gene was raised. In contrast, the expression of the Bcl-2 gene was dramatically diminished. More interestingly, in the current work, Gabapentin treatment significantly decreased the expression of the Bax gene and markedly elevated the expression of the Bcl-2 gene, showing its renal anti-apoptotic efficacy during sepsis induced by CLP. Previous studies reported that Gabapentin has anti-apoptotic activity in diabetic rats' retinas and neuronal injury through stimulating Bcl-2 and repressing Bax expression (Ola et al. 2019; Yan et al. 2019a).
In this work, we revealed the underlying molecular mechanisms by which Gabapentin may exert its nephroprotective effects against CLP-induced sepsis-related AKI in rats. Nevertheless, further research and follow-up examinations are needed to understand more molecular pathways through which Gabapentin may exert its beneficial reno-protective effects against CLP-induced sepsis.
Conclusion
Gabapentin substantially reduced septic-induced renal oxidative stress and inflammation, as shown by the elevation in GSH and SOD levels and the repression in MDA, TNF-α, IL-1β, and IL-6 levels. In addition, it inhibited apoptosis by increasing the expression of Bcl-2 gene and decreasing the expression of Bax gene. Moreover, Gabapentin inhibited the expression of NF-kB gene and activated the Nrf-2/HO-1 signaling pathway. Using the CLP-induced sepsis model in rats, we conclude that the promising nephroprotective potential of Gabapentin may be mediated by modulating the Nrf-2/HO-1, oxidative stress, and apoptosis signaling pathways.
Data availability
All data generated or analysed during this study are included in this published article.
References
Abd El-Baky RM, Hetta HF, Koneru G, Ammar M, Shafik EA, Mohareb DA, Abbas El-Masry M, Ramadan HK, Abu Rahma MZ, Fawzy MA, Fathy M (2020) Impact of interleukin IL-6 rs-1474347 and IL-10 rs-1800896 genetic polymorphisms on the susceptibility of HCV-infected Egyptian patients to hepatocellular carcinoma. Immunol Res 68:118–125
Abdellatef AA, Fathy M, Mohammed AEI, Bakr MSA, Ahmed AH, Abbass HS, El-Desoky AH, Morita H, Nikaido T, Hayakawa Y (2021) Inhibition of cell-intrinsic NF-kappaB activity and metastatic abilities of breast cancer by aloe-emodin and emodic-acid isolated from Asphodelus microcarpus. J Nat Med 75:840–853
Abdelnaser M, Alaaeldin R, Attya ME, Fathy M (2023) Hepatoprotective potential of gabapentin in cecal ligation and puncture-induced sepsis; targeting oxidative stress, apoptosis, and NF-kB/MAPK signaling pathways. Life Sci 320:121562
Abdel-Salam OM, Khadrawy YA, Mohammed NA, Youness ER (2012) The effect of gabapentin on oxidative stress in a model of toxic demyelination in rat brain. J Basic Clin Physiol Pharmacol 23:61–68
Abdelzaher WY, Abdel-Hafez SMN, Rofaeil RR, Ali A, Hegazy A, Bahaa HA (2021) The protective effect of fenofibrate, triptorelin, and their combination against premature ovarian failure in rats. Naunyn Schmiedebergs Arch Pharmacol 394:137–149
Alaaeldin R, Nazmy MH, Abdel-Aziz M, Abuo-Rahma GE-DA, Fathy M (2020) Cell Cycle Arrest and Apoptotic Effect of 7-(4-(N-substituted carbamoylmethyl) piperazin-1-yl) Ciprofloxacin-derivative on HCT 116 and A549 Cancer Cells. Anticancer Res 40:2739–2749
Alaaeldin R, Abuo-Rahma GE-DA, Zhao Q-L, Fathy M (2021) Modulation of apoptosis and epithelial-Mesenchymal transition E-cadherin/TGF-β/Snail/TWIST pathways by a new ciprofloxacin chalcone in breast cancer cells. Anticancer Res 41:2383–2395
Alaaeldin R, Ali FEM, Bekhit AA, Zhao Q-L, Fathy M (2022) Inhibition of NF-kB/IL-6/JAK2/STAT3 Pathway and Epithelial-Mesenchymal Transition in Breast Cancer Cells by Azilsartan. Molecules 27:7825
Alaaeldin R, Bakkar SM, Mohyeldin RH, Ali FE, Abdel-Maqsoud NMR, Fathy M (2023) Azilsartan Modulates HMGB1/NF-κB/p38/ERK1/2/JNK and Apoptosis Pathways during Renal Ischemia Reperfusion Injury. Cells 12:185
Bancroft JD, and Gamble M, (2008) Theory and practice of histological techniques. 6th edn. Churchill Livingstone, Elsevier, China
Bitto A, Minutoli L, David A, Irrera N, Rinaldi M, Venuti FS, Squadrito F, Altavilla D (2012) Flavocoxid, a dual inhibitor of COX-2 and 5-LOX of natural origin, attenuates the inflammatory response and protects mice from sepsis. Crit Care 16:1–12
Brooks HF, Moss RF, Davies NA, Jalan R, Davies DC (2014) Caecal ligation and puncture induced sepsis in the rat results in increased brain water content and perimicrovessel oedema. Metab Brain Dis 29:837–843
Brown MA, Jones WK (2004) NF-kappaB action in sepsis: the innate immune system and the heart. Front Biosci-Landmark 9:1201–1217
Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, Reddy R, Epperson CN (2012) The impact of gabapentin administration on brain GABA and glutamate concentrations: a 7T 1H-MRS study. Neuropsychopharmacology 37:2764–2771
Cai Z, Sheng Z, Yao H (2017) Pachymic acid ameliorates sepsis-induced acute kidney injury by suppressing inflammation and activating the Nrf2/HO-1 pathway in rats. Eur Rev Med Pharmacol Sci 21:1924–1931
Chittenden T, Harrington EA, O’Connor R, Remington C, Lutz RJ, Evan GI, Guild BC (1995) Induction of apoptosis by the Bcl-2 homologue Bak. Nature 374:733–736
de Brito TV, Júnior GJD, da Cruz Júnior JS, Silva RO, da Silva Monteiro CE, Franco AX, Vasconcelos DFP, de Oliveira JS, da Silva Costa DV, Carneiro TB (2020) Gabapentin attenuates intestinal inflammation: role of PPAR-gamma receptor. Eur J Pharmacol 873:172974
El-Emam SZ, Soubh AA, Al-Mokaddem AK, Abo El-Ella DM (2020) Geraniol activates Nrf-2/HO-1 signaling pathway mediating protection against oxidative stress-induced apoptosis in hepatic ischemia-reperfusion injury. Naunyn Schmiedebergs Arch Pharmacol 393:1849–1858
Fathy M, Khalifa E, Fawzy MA (2019) Modulation of inducible nitric oxide synthase pathway by eugenol and telmisartan in carbon tetrachloride-induced liver injury in rats. Life Sci 216:207–214
Fawzy MA, Maher SA, Bakkar SM, El-Rehany MA, Fathy M (2021) Pantoprazole Attenuates MAPK (ERK1/2, JNK, p38)-NF-kappaB and Apoptosis Signaling Pathways after Renal Ischemia/Reperfusion Injury in Rats. Int J Mol Sci 22:10669
Fawzy MA, Maher SA, El-Rehany MA, Welson NN, Albezrah NKA, Batiha GE-S, Fathy M (2022) Vincamine Modulates the Effect of Pantoprazole in Renal Ischemia/Reperfusion Injury by Attenuating MAPK and Apoptosis Signaling Pathways. Molecules 27:1383
Fawzy MA, Beshay ON, Bekhit AA, Abdel-Hafez SMN, Batiha GE-S, Bin Jardan YA, Fathy M (2023a) Nephroprotective effect of AT-MSCs against cisplatin-induced EMT is improved by azilsartan via attenuating oxidative stress and TGF-β/Smad signaling. Biomed Pharmacother 158:114097
Fawzy MA, Nasr G, Ali FEM, Fathy M (2023b) Quercetin potentiates the hepatoprotective effect of sildenafil and/or pentoxifylline against intrahepatic cholestasis: Role of Nrf2/ARE, TLR4/NF-κB, and NLRP3/IL-1β signaling pathways. Life Sci 314:121343
Galley HF (2011) Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth 107:57–64
Gómez H, Kellum JA (2019) Sepsis-induced acute kidney injury. Critical Care Nephrology 22:524–533 (e523)
Gonçalves GM, Zamboni DS, Câmara NOS (2010) The role of innate immunity in septic acute kidney injuries. Shock 34:22–26
Gordh TE, Stubhaug A, Jensen TS, Arnèr S, Biber B, Boivie J, Mannheimer C, Kalliomäki J, Kalso E (2008) Gabapentin in traumatic nerve injury pain: a randomized, double-blind, placebo-controlled, cross-over, multi-center study. Pain 138:255–266
Höcherl K, Schmidt C, Kurt B, Bucher M (2010) Inhibition of NF-κB ameliorates sepsis-induced downregulation of aquaporin-2/V2 receptor expression and acute renal failure in vivo. Am J Physiol Renal Physiol 298:F196–F204
Ibrahim YF, Moussa RA, Bayoumi A, Ahmed A-SF (2020) Tocilizumab attenuates acute lung and kidney injuries and improves survival in a rat model of sepsis via down-regulation of NF-κB/JNK: a possible role of P-glycoprotein. Inflammopharmacology 28:215–230
Li W, Khor TO, Xu C, Shen G, Jeong W-S, Yu S, Kong A-N (2008) Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76:1485–1489
Lin Z, Jin J, Shan X (2019) Fish oils protects against cecal ligation and puncture-induced septic acute kidney injury via the regulation of inflammation, oxidative stress and apoptosis. Int J Mol Med 44:1771–1780
Lopes JA, Fernandes P, Jorge S, Resina C, Santos C, Pereira Á, Neves J, Antunes F, Gomes da Costa A (2010) Long-term risk of mortality after acute kidney injury in patients with sepsis: a contemporary analysis. BMC Nephrol 11:1–10
Luo C-J, Luo F, Bu Q-D, Jiang W, Zhang W, Liu X-M, Che L, Luan H, Zhang H, Ma R-X (2020) Protective effects of resveratrol on acute kidney injury in rats with sepsis. Biomed Pap Med Fac Palacky Univ Olomouc 164:49–56
Macdonald SP, Williams JM, Shetty A, Bellomo R, Finfer S, Shapiro N, Keijzers G (2017) Sepsis in the emergency department–Part 1: Definitions and outcomes. Emerg Med Australas 29:619–625
Maneuf YP, Luo ZD, Lee K (2006) alpha2delta and the mechanism of action of gabapentin in the treatment of pain. Semin Cell Dev Biol 17(5):565-570. https://doi.org/10.1016/j.semcdb.2006.09.003
Motavallian A, Bouzari S, Zamani E, Karimian P, Dabirian S, Molavi M, Torshkooh FA (2021) An investigation of the anti-inflammatory effects of gabapentin on acetic acid-induced colitis in rats. Mol Biol Rep 48:3423–3430
Ola MS, Alhomida AS, LaNoue KF (2019) Gabapentin attenuates oxidative stress and apoptosis in the diabetic rat retina. Neurotox Res 36:81–90
Park S-D, Cheon SY, Park T-Y, Shin B-Y, Oh H, Ghosh S, Koo B-N, Lee S-K (2015) Intranuclear interactomic inhibition of NF-κB suppresses LPS-induced severe sepsis. Biochem Biophys Res Commun 464:711–717
Requena JR, Fu M-X, Ahmed MU, Jenkins AJ, Lyons TJ, Thorpe SR (1996) Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol Dial Transplant 11:48–53
Ritter C, Andrades M, Frota MLC, Bonatto F, Pinho RA, Polydoro M, Klamt F, Pinheiro CT, Menna-Barreto SS, Moreira JCF (2003) Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Med 29:1782–1789
Sabra RT, Abdellatef AA, Abdel-Sattar E, Fathy M, Meselhy MR, Hayakawa Y (2022) Russelioside A, a Pregnane Glycoside from Caralluma tuberculate, Inhibits Cell-Intrinsic NF-κB Activity and Metastatic Ability of Breast Cancer Cells. Biol Pharm Bull 45:1564–1571
Samra YA, Amin MN, Said E (2021) Cardio-protective impact of gabapentin against doxorubicin-induced myocardial toxicity in rats; emphasis on modulation of inflammatory-apoptotic signaling. Int Immunopharmacol 90:107125
Shytaj IL, Fares M, Gallucci L, Lucic B, Tolba MM, Zimmermann L, Adler JM, Xing N, Bushe J, Gruber AD, Ambiel I, Taha Ayoub A, Cortese M, Neufeldt CJ, Stolp B, Sobhy MH, Fathy M, Zhao M, Laketa V, Diaz RS, Sutton RE, Chlanda P, Boulant S, Bartenschlager R, Stanifer ML, Fackler OT, Trimpert J, Savarino A, Lusic M (2022) The FDA-Approved Drug Cobicistat Synergizes with Remdesivir To Inhibit SARS-CoV-2 Replication In Vitro and Decreases Viral Titers and Disease Progression in Syrian Hamsters. mBio 13:e0370521
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810
Surh Y-J, Na H-K (2008) NF-κB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals. Genes Nutr 2:313–317
Vomund S, Schäfer A, Parnham MJ, Brüne B, Von Knethen A (2017) Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci 18:2772
Wang Q, Zhang L, Yuan X, Ou Y, Zhu X, Cheng Z, Zhang P, Wu X, Meng Y, Zhang L (2016) The relationship between the Bcl-2/Bax proteins and the mitochondria-mediated apoptosis pathway in the differentiation of adipose-derived stromal cells into neurons. PLoS ONE 11:e0163327
Wang Y, Feng F, Liu M, Xue J, Huang H (2018) Resveratrol ameliorates sepsis-induced acute kidney injury in a pediatric rat model via Nrf2 signaling pathway. Exp Ther Med 16:3233–3240
Wu G-J, Lin Y-W, Chuang C-Y, Tsai H-C, Chen R-M (2018) Liver nitrosation and inflammation in septic rats were suppressed by propofol via downregulating TLR4/NF-κB-mediated iNOS and IL-6 gene expressions. Life Sci 195:25–32
Yan BC, Wang J, Rui Y, Cao J, Xu P, Jiang D, Zhu X, Won M-H, Bo P, Su P (2019a) Neuroprotective effects of gabapentin against cerebral ischemia reperfusion-induced neuronal autophagic injury via regulation of the PI3K/Akt/mTOR signaling pathways. J Neuropathol Exp Neurol 78:157–171
Yan X-X, Zheng A-D, Zhang Z-E, Pan G-C, Zhou W (2019b) Protective effect of pantoprazole against sepsis-induced acute lung and kidney injury in rats. Am J Transl Res 11:5197
Yerra VG, Negi G, Sharma SS, Kumar A (2013) Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol 1:394–397
Yosri H, Said E, Elkashef WF, Gameil NM (2018) Modulatory role of gabapentin against ovalbumin-induced asthma, bronchial and airway inflammation in mice. Environ Toxicol Pharmacol 64:18–25
Zaki MYW, Fathi AM, Samir S, Eldafashi N, William KY, Nazmy MH, Fathy M, Gill US, Shetty S (2022) Innate and Adaptive Immunopathogeneses in Viral Hepatitis; Crucial Determinants of Hepatocellular Carcinoma. Cancers 14:1255
Zarjou A, Agarwal A (2011) Sepsis and acute kidney injury. J Am Soc Nephrol 22:999–1006
Zhang Z, Liang Z, Li H, Li C, Yang Z, Li Y, She D, Cao L, Wang W, Liu C (2017) Perfluorocarbon reduces cell damage from blast injury by inhibiting signal paths of NF-κB, MAPK and Bcl-2/Bax signaling pathway in A549 cells. PLoS ONE 12:e0173884
Zhang N, Zhao W, Hu Z-J, Ge S-M, Huo Y, Liu L-X, Gao B-L (2021) Protective effects and mechanisms of high-dose vitamin C on sepsis-associated cognitive impairment in rats. Sci Rep 11:1–10
Acknowledgements
Special thanks and great appreciation to Alaa Al-Kadi, Assistant Lecturer of Pharmacology & Toxicology, Faculty of Pharmacy, Deraya University, Egypt, for her assistance in the CLP surgery procedures.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
R.A. and M.F. designed and supervised the study. M.A., R.A., and M.E.A. performed the experiments. All authors analyzed the data and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
All animal experiments and care were conducted according to Minia University, College of Pharmacy's Research Ethics Committee (license number: ES 07/2021).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelnaser, M., Alaaeldin, R., Attya, M.E. et al. Modulating Nrf-2/HO-1, apoptosis and oxidative stress signaling pathways by gabapentin ameliorates sepsis-induced acute kidney injury. Naunyn-Schmiedeberg's Arch Pharmacol 397, 947–958 (2024). https://doi.org/10.1007/s00210-023-02650-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02650-y