Abstract
Dysfunction of the lower urinary tract (LUT) including urinary bladder and urethra (and prostate in men) is one of the most frequent complications of diabetes and can manifest as overactive bladder, underactive bladder, urinary incontinence, and as aggravated symptoms of benign prostate hyperplasia. We have performed a selective literature search to review existing evidence on efficacy of classic medications for the treatment of LUT dysfunction in diabetic patients and animals, i.e., α1-adrenoceptor and muscarinic receptor antagonists, β3-adrenoceptor agonists, and phosphodiesterase type 5 inhibitors. Generally, these agents appear to have comparable efficacy in patients and/or animals with and without diabetes. We also review effects of antidiabetic medications on LUT function. Such studies have largely been performed in animal models. In the streptozotocin-induced models of type 1 diabetes, insulin can prevent and reverse alterations of morphology, function, and gene expression patterns in bladder and prostate. Typical medications for the treatment of type 2 diabetes have been studied less often, and the reported findings are not yet sufficient to derive robust conclusions. Thereafter, we review animal studies with emerging medications perhaps targeting diabetes-associated LUT dysfunction. Data with myoinositol, daidzein, and with compounds that target oxidative stress, inflammation, Rac1, nerve growth factor, angiotensin II receptor, serotonin receptor, adenosine receptor, and soluble guanylyl cyclase are not conclusive yet, but some hold promise as potential treatments. Finally, we review nonpharmacological interventions in diabetic bladder dysfunction. These approaches are relatively new and give promising results in preclinical studies. In conclusion, the insulin data in rodent models of type 1 diabetes suggest that diabetes-associated LUT function can be mostly or partially reversed. However, we propose that considerable additional experimental and clinical studies are needed to target diabetes itself or pathophysiological changes induced by chronic hyperglycemia for the treatment of diabetic uropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a disease of high and further growing global prevalence (World Health Organization 2016). It leads to complications in the cardiovascular, renal, and ocular system that cause major morbidity and mortality and, thereby, constitute a major challenge to the healthcare system (Harding et al. 2019). Diabetes is also associated with an impaired function of lower urinary tract (LUT), including the bladder and urethra (and prostate in men) (Liu and Daneshgari 2014). LUT dysfunction (LUTD) often leads to LUT symptoms (LUTS) encompassing storage, voiding, or post micturition symptoms. LUTD in general and urinary bladder dysfunction are estimated to occur in 80% and 50% of diabetic patients, respectively (Daneshgari and Moore 2006; Daneshgari et al. 2009). The presence of diabetes is associated with a greater likelihood of experiencing LUTD and with a greater severity of such dysfunction. For instance, the average severity of LUTS associated with benign prostatic enlargement increases with age and they are worse in diabetic patients than in those without, i.e., a man with diabetes mathematically has worse symptoms comparable to those of a man without diabetes but 12 years older (Michel et al. 2000). Diabetes also is associated with a greater reduction of nocturia-related quality of life in men with LUTS attributed to an enlarged prostate (Michel et al. 2020).
Data from animal models of acquired diabetes support the idea that the above associations represent cause-effect relationships. Experimental diabetes can cause LUTD including overactive bladder syndrome (OAB) or, in advanced/late stages of the condition, underactive bladder syndrome (UAB) (Liu and Daneshgari 2014; Oger-Roussel et al. 2014; Tatemichi et al. 2015; Masuda et al. 2020). Similarly, numerous studies have reported an enlargement of the urinary bladder in animal models of type 1 DM (T1DM) (Arioglu Inan et al. 2018); a similar enlargement occurs in some but not other animal models of type 2 DM (T2DM) (Ellenbroek et al. 2018). A reduced size of the prostate was found in multiple studies in T1DM animals (Latifpour et al. 1991; Fukumoto et al. 1993; Wang et al. 2000; Yono et al. 2005), whereas a decrease (Atalay et al. 2017) or increase (Elabbady et al. 2016) was observed in T2DM patients, depending on the blood glucose levels.
Diabetes-associated LUTD could be managed by four, not mutually exclusive approaches: established treatments of LUT dysfunction assuming that they are similarly effective in patients with and without diabetes; antidiabetic treatments assuming that a normalization of glucose levels will also normalize LUT morphology and function; emerging treatments of LUTD by targeting pathophysiological changes in diabetes; and nonpharmacological interventions for individuals in whom pharmacological treatment is not effective. These options will be discussed hereafter based on a selective literature search and are summarized in Fig. 1.
Effects of established medications on LUTD in diabetes
Guideline-recommended treatments of LUTD include α1-adrenoceptor antagonists, 5α-reductase inhibitors, and phosphodiesterase type 5 (PDE5) inhibitors (particularly in the context of benign prostatic enlargement) (Oelke et al. 2012) and muscarinic receptor antagonists and β3-adrenoceptor agonists (particularly in the context of OAB) (Gratzke et al. 2015); some of these have been evaluated in diabetic patients as summarized in Table 1. In most cases, clinical comparison of effects between diabetic and nondiabetic patients is based on exploratory and often post hoc analysis of studies primarily designed for other purposes. Some animal studies were designed to explore effects of medications on diabetes-associated LUTD.
α1-Adrenoceptor antagonists
Clinical and experimental studies have tested α1-adrenoceptor antagonists in diabetic patients and animals. A large noninterventional study in men with LUTS compared beneficial effects of tamsulosin in 1290 men with and 8566 without diabetes (Michel et al. 2000). While tamsulosin reduced symptom severity assessed as International Prostate Symptom Score (IPSS) by 10.5 points in the overall group, a diagnosis of diabetes was associated with a 0.7 points smaller reduction in a multivariate analysis. This may be of limited clinical relevance because a symptom change of at least 3 points is assumed to be noticeable by a patient (Barry et al. 1995). A retrospective analysis of men with LUTS treated with alfuzosin, doxazosin, tamsulosin, or terazosin with (n = 60) and without diabetes (n = 221) reported reductions in IPSS by 7.5 and 3.1 points, respectively (Bozlu et al. 2004); however, these data are not easily interpretable because diabetics had a greater IPSS at baseline, no baseline adjustments were made, and reported statistical analysis only compared baseline with post-treatment data within each group. Taken together, these data indicate that α1-adrenoceptor antagonists have comparable efficacy in alleviating LUTS to a clinically relevant extent in men with as compared to those without diabetes.
Studies in diabetic animals primarily explored whether α1-adrenoceptor antagonists can improve dysfunction of the bladder and urethra. Most used the streptozotocin (STZ) model of T1DM, but one used Zucker diabetic fatty rats as a model of T2DM (Tatemichi et al. 2015). An increased bladder capacity is a frequent feature of animal models of diabetes (Ellenbroek et al. 2018), apparently reflecting the need to handle increased volumes of urine related to diabetic polyuria. While an increased bladder capacity clinically is more typical for UAB than OAB, it remains unclear whether this alone constitutes proof of an UAB. In STZ-injected rats, chronic administration of silodosin attenuated the diabetes-associated increases in bladder capacity and residual urine (Yonekubo et al. 2017). Acute administration of tamsulosin in the rat STZ model did not affect bladder capacity or residual urine (Sekido et al. 2020), and acute administration of silodosin did not alter bladder capacity or residual volume in Zucker diabetic fatty rats (Tatemichi et al. 2015). Acute administration of terazosin (Torimoto et al. 2005) or tamsulosin (Chen et al. 2014) was reported to improve urethral function in STZ-injected rats, i.e., reduced the elevated urethral perfusion pressure.
5α-Reductase inhibitors
5α-Reductase inhibitors are frequntly used in the treatment of LUTS suggestive of benign prostatic hyperplasia (BPH). Little clinical evidence is available on 5α-reductase inhibitor effects in DM. In population-based cohort studies from the UK and Taiwan, use of a 5α-reductase inhibitors was associated with a greater risk to develop new-onset T2DM as compared to use of tamsulosin; the hazard ratios for dutasteride and finasteride relative to tamsulosin were 1.32 [95% confidence interval 1.08; 1.61] and 1.26 [1.10; 1.45], respectively, in the UK with similar numbers in Taiwan (Wei et al. 2019). The mechanistic plausibility of these findings is supported by two animal studies: 5α-reductase knock-out mice and Zucker diabetic fatty rats treated with finasteride reported that such inhibition predisposed to insulin resistance (Livingstone et al. 2015). While we did not identify studies comparing the efficacy of 5α-reductase inhibitors in men with and without diabetes, these data suggest that if anything their use should make the diabetes-associated part of male LUT symptoms worse.
Phosphodiesterase type 5 inhibitors
PDE5 inhibitors are primarily indicated for the treatment of erectile dysfunction. While various PDE5 inhibitors have been tested in phase 1 and 2 studies in LUTS patients, only tadalafil was approved for the treatment of male LUTS attributed to benign prostatic enlargement. While we are not aware of clinical studies comparing its efficacy in men with LUTS with and without concomitant diabetes, a pooled analysis of 11 randomized clinical trials found that its efficacy in the treatment of erectile dysfunction is slightly lower in those with than those without diabetes (34.7% vs. 36.9% attaining normal erectile function) (Lewis et al. 2005), possibly reflecting diabetic neuropathy. In a clinical study in men with LUTS and OAB, diabetes was present as a comorbidity in 10 of 44 responders but in 0 of 9 nonresponders (Matsuo et al. 2020), indicating that the effects of tadalafil and possible other PDE5 inhibitors in LUTS may not be attenuated by concomitant diabetes. Seven weeks after STZ injection in rats, a 7-day treatment with tadalafil restored prolonged inter-contraction intervals and diminished bladder blood flow to values comparable to those in control animals (Gotoh et al. 2018). In a follow-up study, tadalafil restored urethral relaxation and detrusor contraction (Gotoh et al. 2019). Restoration of detrusor function by tadalafil in UAB observed at late time points after administration of STZ was also reported by others (Masuda et al. 2020). These data indicate beneficial effects of tadalafil in diabetic uropathy. Based on the proposed role of diminished perfusion in bladder dysfunction (Michel et al. 2015a; Thurmond et al. 2016), they also provide evidence that the beneficial effects may at least partly be due to improved perfusion.
Muscarinic receptor antagonists
The effect of muscarinic receptor antagonists in patients with OAB has been compared between those with and without concomitant diabetes, largely in noninterventional studies. In male and female OAB patients receiving darifenacin (532 with 1315 without concomitant diabetes), the presence of diabetes was associated with smaller improvements of urgency, incontinence, frequency, and nocturia in a multivariate analysis adjusting for gender, age, and baseline symptoms, but the impact of diabetes was small relative to the overall symptom improvement by darifenacin (Schneider et al. 2013). Two other noninterventional studies have compared the effects of adding a muscarinic receptor antagonist in men with and without diabetes and with persisting storage symptoms despite treatment with an α-blocker. In a cohort of 741 men, addition of tolterodine caused comparable additional improvements in IPSS in men with and without diabetes (Höfner et al. 2010). In a similar study, addition of solifenacin was associated with a smaller improvement of the OAB Symptom Score in a multivariate analysis in the presence of diabetes of a total group of 130 men (0.6 vs. 2.1 points of the OAB Symptom Score) (Obata et al. 2013). One prospective open-label study compared effects of solifenacin in 81 women with diabetes-associated OAB with those in 160 women with idiopathic OAB and found a comparable degree of symptom improvement in both groups (Choi et al. 2018). While small sample sizes make interpretation of the data difficult, it appears that muscarinic antagonists may be slightly less effective in those with OAB symptoms with as compared to those without concomitant diabetes. However, group differences appear to be small, and the efficacy of the muscarinic antagonists in the diabetic groups remained of clinical relevance. Animal studies support this conclusion: Goto-Kakizaki rats, a T2DM model, exhibited detrusor overactivity that was markedly reduced by solifenacin (Oger-Roussel et al. 2014). An interesting alternative explanation for slightly smaller efficacy of the OAB medications in those with diabetes relates not to pharmacodynamic differences but rather to a reported lesser adherence to muscarinic antagonists prescribed as OAB medication in obese patients (Lua et al. 2017), a feature common in T2DM. On the other hand, analysis of a large database has found that treatment with a muscarinic antagonist moderately selective for M3 receptors such as darifenacin or solifenacin as compared to oxybutynin increased the risk for developing diabetes with a hazard ratio of 1.57 [1.48; 1.67] and 1.29 [1.24; 1.35], respectively (Selig et al. 2020); these data do not allow conclusions whether darifenacin or solifenacin promote diabetes or protect less efficiently than oxybutynin; the impact on diabetes-associated LUT dysfunction remains to be assessed.
β3-Adrenoceptor agonists
While we are not aware of clinical data with a β3-adrenoceptor agonist in OAB patients with and without diabetes, chronic ischemia/hypoxia is proposed to play a pathophysiological role in bladder dysfunction (Michel et al. 2015a; Thurmond et al. 2016). Against this background, a study reported that treatment with the experimental β3-adrenoceptor agonist CL 316,243 attenuated vascular damage from ischemia/reperfusion injury in mice and in isolated human endothelial cells (Bubb et al. 2021). Although brown adipose tissue is present in humans to a limited extent only, administration of the β3-adrenoceptor agonist mirabegron enhanced thermogenesis in humans (Cypess et al. 2015), an effect that if anything should improve glycemic control. More recent work from the same group found that mirabegron also improved insulin sensitivity in healthy volunteers (O'Mara et al. 2020). Whether such effects also relate to bladder dysfunction remains to be established.
Effects of antidiabetic treatments on LUTD in diabetes
Not all complications of diabetes are equally well prevented by glucose lowering (Beckman and Creager 2016). This raises the question whether antidiabetic medications substantially improve LUT function. Conceptually, this could be a reversal of dysfunction in established diabetes or a prevention of dysfunction in new onset diabetes (operationally defined as starting within one week after start of diabetes); the latter is not a realistic clinical setting with the possible exception of T1DM. We are unaware of clinical studies exploring the effect of antidiabetic medication on LUT function in a treatment or prevention setting, but various animal studies have been reported in both settings. A key feature of LUT manifestations of diabetes in experimental animals is an enlargement of the urinary bladder; this is consistently found in all animal models of T1DM, and in some but not others of T2DM (Arioglu Inan et al. 2018; Ellenbroek et al. 2018). Therefore, many experimental studies have reported bladder weight as outcome parameter for antidiabetic treatment effects on LUT function. Most studies have applied insulin in the T1DM model of STZ-induced diabetes (mostly in rats), whereas information on treatments typically used in the management of T2DM is emerging only recently.
Insulin
Only limited clinical data are available for the effect of insulin on bladder dysfunction in diabetic individuals. The Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study participants were evaluated with follow-up studies for the development of urinary system complications. In a 10-year follow-up study, LUTS were evaluated in 591 men with a T1DM history for ~ 20 years. One hundred fifteen men (mean 44.6 ± 6.6 years of age) reported to have moderate/severe LUTS. The severity and prevalence of LUTS markedly increased after age of 50 s; however, blood glucose control with either intensive (3 or more times per day or continuous injection with insulin pump) or conventional (1 or 2 injection per day) insulin treatment did not affect the severity of LUTS of the cohort (Van Den Eeden et al. 2009). However, considering the greater prevalence of LUTS in older ages (Irwin et al. 2006), it is not plausible to extrapolate this finding to the general population. In a 10- and 17-year follow-up study, urinary incontinence (at least once a week) in 471 women with T1DM was evaluated as an incident reported at 17-year but not at 10-year follow-up questionnaire. Sixty-four women developed urinary incontinence in a 7-year time period. Mean HbA1c levels up to EDIC year 10 were higher (8.4 ± 1.2% vs 7.9 ± 1.1%) in women who developed urinary incontinence compared with the women who did not develop urinary incontinence. Lower HbA1c levels were associated with a reduced odds ratio of urinary incontinence incident (Lenherr et al. 2016) whereas an earlier study revealed no association between HbA1c levels and incontinence in women with both T1DM and T2DM (Lee et al. 2013). Thus, these clinical data allow only limited conclusions on the effect of treatment with insulin on LUTS in diabetic patients.
Considerably more information is available from the STZ model of T1DM in rats. The high glucose levels following injection of STZ typically lead to polyuria caused by osmotic diuresis. Treatment with insulin consistently reduced diabetic polyuria in a prevention (Longhurst et al. 1991; Daneshgari et al. 2006; Melman et al. 2009; Gotoh et al. 2021) and in a treatment setting (Longhurst et al. 1991; Xiao et al. 2015). This is relevant because some investigators proposed that polyuria is the main cause of bladder enlargement in experimental diabetes, although some analyses did not confirm that (Ellenbroek et al. 2018; Yesilyurt et al. 2019). The effect of insulin treatment on bladder weight has mostly been studied in a prevention setting, i.e., starting within 1 week after induction diabetes. With one exception (Evcim et al. 2015), these studies consistently reported that early administration of insulin in a dose to avoid major elevations in blood glucose prevented or at least strongly attenuated diabetes-associated bladder enlargement (Longhurst et al. 1991; Yono et al. 2005; Christ et al. 2006; Daneshgari et al. 2006; Melman et al. 2009). Similarly, a more limited number of studies in a treatment setting reported that insulin administration starting 3 (Xiao et al. 2015) or 8 weeks after STZ injection (Longhurst et al. 1991; Fukumoto et al. 1994), i.e., at a time when bladder enlargement had fully developed (Arioglu Inan et al. 2018), largely reversed bladder enlargement. Interestingly, these reversal data indicate that bladder denervation, possibly occurring as part of diabetic polyneuropathy, may not be a main cause of bladder enlargement as nerve regeneration is not expected within the time frames in which insulin could reverse bladder enlargement.
The in vivo analysis of bladder function in diabetes has been based on experiments using metabolic cages (Longhurst et al. 1991; Daneshgari et al. 2006; Xiao et al. 2015; Gotoh et al. 2021) and/or cystometry (Christ et al. 2006; Daneshgari et al. 2006; Melman et al. 2009; Xiao et al. 2015; Gotoh et al. 2021). In a treatment (Xiao et al. 2015) and in a prevention design (all other studies), it was observed that insulin reduced number of micturitions and mean voided volume per micturition, which is to be expected if insulin lowers glucose levels and thereby reduces diabetic polyuria. Various cystometric parameters including bladder capacity were also improved. Insulin also prevented the diabetes-associated increases in residual urine (Christ et al. 2006; Daneshgari et al. 2006; Wang et al. 2010). The effects of treatment with insulin on in vitro parameters of bladder function have been studied less often and yielded conflicting results: contractile responses to a muscarinic agonist were increased in diabetes, but this was attenuated in strips from insulin-treated animals in one (Longhurst et al. 1991) but not another study (Evcim et al. 2015). Of note, the Longhurst study consistently observed an improvement with insulin administered both in a prevention and a treatment setting and reported similar findings if ATP was used as the contractile agonist. One group reported that STZ alone increased expression of muscarinic receptors in the bladder and that insulin starting early after STZ administration prevented that (Latifpour et al. 1992). In a follow-up study, they reported that insulin administration starting 8 weeks after STZ also attenuated the increased expression of muscarinic receptors (Fukumoto et al. 1994). In a similarly designed study, they also reported that insulin reversed the STZ-induced upregulation of endothelin receptors in the bladder (Saito et al. 2000b). Another study found that diabetes lasting for 6 or 10 weeks led to altered length/tension curves (Starling mechanism) and that was prevented by treatment with insulin (Wang et al. 2010). Finally, it was found that treatment with insulin can also prevent diabetes-associated dysfunction of the urethra (Gotoh et al. 2021).
Over a period of two decades, a group from New Haven, CT, has continuously explored the effects of treatment with insulin on STZ effects in the rat prostate and, to a lesser extent vas deferens. In their initial experiments, they started insulin treatment 3 days after injection of STZ. This caused the expected increase in urine output; however, in contrast to findings in the bladder (Arioglu Inan et al. 2018), this did not enlarge but rather reduce prostate weight to an extent at least as much as the reduction in body weight, the latter being a typical feature of STZ administration. This was accompanied by a major reduction in the density of muscarinic receptors (Latifpour et al. 1991) and of β-adrenoceptors (Gousse et al. 1991) as detected in radioligand binding studies. Treatment with insulin prevented all of these alterations. Of note, the radioligand used for detection of β-adrenoceptors, [3H]-dihydroalprenolol, was used in concentrations that allow detection of β1- and β2-, but not β3-adrenoceptors (Niclauß et al. 2006). Apparently, the detected receptors were an almost homogenous population of β2-adrenoceptors in all three groups. The muscarinic receptors being detected apparently largely belonged to the M3 subtype in all three groups, whereas several other groups reported a presence of mainly M1 receptors in the prostate (Witte et al. 2008). A follow-up study used the same model but started treatment with insulin 8 weeks after induction of diabetes. When measured 16 weeks after administration of STZ (8 weeks after start of insulin treatment), prostate weight was also reduced, which was accompanied by reduced serum testosterone levels (Fukumoto et al. 1993). Treatment with insulin reversed these changes and the reduction of muscarinic and β-adrenergic binding sites. Applying the same study design, the weight of the vas deferens was also reduced whereas the density of muscarinic binding sites in this tissue was increased in insulin-treated animals (Kamai et al. 1994). The affinity estimates for multiple muscarinic antagonists in prostate and vas deferens were correlated only poorly, indicating that the difference between the two tissues may reflect a different dominant subtype of muscarinic receptors. In an additional study of similar design, treatment with insulin also reversed the upregulation of endothelin-1 binding sites in the vas deferens, apparently reflecting a homogenous population ETA receptors (Saito et al. 1996). Subsequent studies also using a treatment design reported that treatment with insulin reversed greater expression of fibroblast growth factor-2 (also known as basic fibroblast growth factor) (Wang et al. 2000), greater expression of transforming growth factor-β (TGF-β), and the related mRNAs, TGF-β1 and TGF-β2 (Ikeda et al. 2000), and increased expression of endothelin receptors (about 80% ETA and 20% ETB receptors in all groups) (Saito et al. 2000a). BB/Wor rats spontaneously develop T1DM. Studies in 8, 16, and 32-week BB/Wor rats as well as those in 32-week-old BB/Wor rats additionally injected with STZ found that diabetes increased the size of the urinary bladder, the vas deferens, the ureter, and, to a lesser extent, the kidney whereas it reduced the size of the ventral prostate and did not change sizes of the dorsolateral prostate, seminal vesicles, testes, or adrenal glands; all diabetes-associated changes were attenuated to some degree by insulin administration dosed to maintain euglycemic levels (Yono et al. 2005). Extending the work in BB/Wor- and STZ-injected BB/Wor rats, the same group also reported on microarray analysis (Yono et al. 2008). The hyperglycemic state was associated with altered expression levels of 856 genes, of which 35 were related to cell growth, proliferation, and cell death in the ventral prostate; such alterations were largely attenuated upon treatment with insulin.
Taken together, these data support the idea that in the STZ-induced model of T1DM, treatment with insulin can largely prevent and reverse diabetes-associated LUT dysfunction, organ sizes, and expression levels of various genes. The importance of these findings is that they establish that the diabetes-associated alterations are largely reversible within certain period of diabetes duration. Moreover, they highlight that tissues within the LUT, most importantly bladder and prostate, exhibit differential regulation by diabetes. A key limitation of these findings is that they are based on an animal model of T1DM, mostly STZ-injected rats. Alterations of bladder size in animal models of T2DM apparently are limited to some models, and the occurrence of bladder enlargement in these models apparently is not related to the degree of hyperglycemia (Ellenbroek et al. 2018). This raises the question whether such changes also be prevented or reversed by treatments other than insulin; such data have only emerged recently.
Metformin
Metformin is the cornerstone of medical treatment of T2DM. It has been studied in some animal models of LUT dysfunction, but interestingly not those of diabetes. In a rat model of testosterone-induced prostate enlargement, treatment with metformin at least partly prevented increases in prostate size (Mosli et al. 2015). It also prevented testosterone-induced increases in estrogen receptor-α and decreases in estrogen receptor-β expression, while not affecting expression of the androgen receptor or of 5α-reductase. Other investigators used rats with partial bladder outlet obstruction and started metformin treatment within 3 days after induction (Chen et al. 2021). In the early phase (2 weeks after induction), metformin lowered baseline bladder pressure and reduced inflammatory reactions. In the chronic phase (9 weeks after induction), metformin improved the reduced bladder compliance, ameliorated fibrosis, and inhibited autophagy. In contrast to these two studies using a prevention design, other investigators induced bladder dysfunction in mice by administering methylglyoxal, a reactive carbonyl species present in high concentrations in the blood of diabetic patients; metformin was administered in the final 2 weeks of a 12-week exposure to methylglyoxal, i.e., in a treatment design (Oliveira et al. 2021). This model is not associated with changes in body weight, water consumption, or blood glucose levels, either in the absence or presence of metformin. However, it is associated with a reduced bladder weight that was not affected by metformin. Nonetheless, metformin reversed elevations in serum methylglyoxal and advanced glycation end products. It also reversed increases in urothelial thickness and collagen content (detrusor thickness was not altered in this model), in total voided volume, volume per void, basal pressure, nonvoiding contractions, bladder capacity, and residual urine leading to an increased voiding efficiency. It reversed increased contractile responses to αβ-methylene-ATP and electrical field stimulation but did not affect reduced contractile responses to carbachol. Taken together, these three studies demonstrate that metformin can have beneficial effects in euglycemic animal models of LUT dysfunction; however, they do not allow conclusions on possible effects in animal models of diabetes.
Sodium-glucose transporter 2 inhibitors
Sodium-glucose transporter 2 (SGLT2) inhibitors such as empagliflozin (Michel et al. 2015b) are an innovative class of oral antidiabetic agents that not only lower glucose levels but also have nephroprotective effects (Wanner et al. 2018) and reduce mortality in diabetic and nondiabetic heart failure patients with a reduced ejection fraction (McDonagh et al. 2022). We have tested the SGLT2 inhibitor dapagliflozin in a randomized, blinded study in a rat model of T2DM that is based on a combination of low-dose STZ injection with a high fat diet (HFD) (Yesilyurt et al. 2019). The study exposed 5-week-old rats to a HFD and an injection of STZ after 4–5 weeks on that diet; a minimum of 6 weeks after the STZ injection, treatment with dapagliflozin started and was maintained for another 12–15 weeks. Interpretation of this study is hampered by the fact that mean glucose levels peaked around 300 mg/dl but declined to 205 mg/dl in the diabetic group at study end. The effectiveness of dapagliflozin was confirmed by an increase in diuresis. Under these conditions of only mild hyperglycemia, the investigators did not observe major changes in bladder weight, or contractile responses to carbachol or KCl, or relaxant responses to several general or β2- or β3-adrenoceptor-selective agonists or forskolin. While not approved for the treatment of T1DM, SGLT2 inhibitors have shown beneficial effects in animal models of T1DM (Michel et al. 2015b). Therefore, effects of empagliflozin were studied in STZ-injected rats, in a preventive setting in female rats (Yesilyurt et al. 2021), and a treatment setting in male rats (Michel et al. 2021); the latter study also tested the dipeptidyl-peptidase 4 inhibitor linagliptin, a drug not expected to affect glucose homeostasis in this model. In the preventive study, empagliflozin lowered glucose levels, albeit not to normoglycemic levels, and prevented increases in urine output and bladder weight. Contractile responses to carbachol or KCl measured in isolated bladder strips or relaxant responses to general or β2- or β3-adrenoceptor-selective agonists or forskolin were not substantially affected by diabetes or empagliflozin. While the treatment study found a similar decrease of blood glucose by empagliflozin (not by linagliptin) as in the prevention study, STZ-associated increases in bladder weight were not affected by either drug. Whether the differences between the two studies reflects those in prevention vs. treatment setting, those between female and male rats or unknown other causes remains unclear. The effect of SGLT2 inhibitors on human LUT function remains to be established.
Dipeptidyl-peptidase 4 inhibitors and glucagon-like peptide 1 receptor agonists
Chronic hypoperfusion due to atherosclerosis of pelvic arteries is considered as a cause of bladder dysfunction (Michel et al. 2015a; Thurmond et al. 2016). One study explored effects of a 4-week treatment with the dipeptidyl-peptidase 4 inhibitor anagliptin and the glucagon-like peptide 1 (GLP-1) receptor agonist liraglutide in a preventive setting in a rat model of acute bilateral internal iliac artery ligation (Hotta et al. 2020). Neither the ligation nor the treatments affected glucose levels or bladder weight. The ligated animals exhibited the expected reductions in bladder blood flow, which were improved with anagliptin. Inter-contraction intervals in cystometric studies were longer in ligated and ligated animals treated with liraglutide, but ligated rats treated with anagliptin exhibited inter-contraction intervals as sham-operated animals. These data indicate that dipeptidyl-peptidase 4 inhibitors may have beneficial effects on bladder function in normoglycemic animals. Other investigators tested the GLP-1 analogs exendin-4 and liraglutide in an acute mouse model of neurogenic voiding dysfunction induced by middle cerebral artery occlusion, in this case in diabetic db/db mice (Li et al. 2016). While this study did not assess glucose levels after ligation and/or any of the treatments, db/db mice are an established a model of T2DM (Kong et al. 2013). The ligation led to a reduced number of voids per 10-min period and an increase in number of nonvoiding contractions of the bladder; when applied in a prevention design, exendin-4 and liraglutide attenuated both effects of ligation. Although the animals being studied apparently were diabetic, this model probably reflects neurogenic dysfunction in diabetic animals and not diabetes-associated LUT dysfunction. Effects of dipeptidyl-peptidase 4 inhibitors and GLP-1 analogs in diabetes-associated bladder dysfunction remain to be studied.
Emerging pathophysiology-based treatments of LUTD in diabetes
Other than general LUTD or antidiabetic drugs, a third possible approach would be medications specifically addressing diabetes-associated pathophysiology in LUTD. The theoretical feasibility of this approach is demonstrated by drugs specifically targeting diabetic retinopathy (Beckman and Creager 2016). Candidates for the treatment of diabetic uropathy are discussed hereunder.
Oxidative stress
Oxidative stress may be one of the underlying mechanisms in diabetic bladder dysfunction as well as other complications of the diabetes (Wang et al. 2016). Multiple studies have reported that oxidative stress increases in bladder tissues in diabetes (Changolkar et al. 2005; Kanika et al. 2011; Ha et al. 2013; Chen et al. 2015; Elrashidy and Liu 2019; Lin et al. 2020; Wang et al. 2021; Laddha and Kulkarni 2022). Oxidative stress has also been reported from clinical studies of various types of LUTD other than diabetes (Antunes-Lopes et al. 2019; Matsuo et al. 2020), and the urinary presence of oxidative stress indicators such as 8-hydroxy-2′-doexyguanosine has been proposed as biomarker for LUTS in general (Kageyama et al. 2018; Matsumoto et al. 2019). Interestingly, treatment for 14 days with the antioxidants resveratrol (10 mg/kg/day, p.o.) and taurine (1 g/kg/day, p.o.) moderately reduced blood glucose levels but almost fully prevented diabetes-associated bladder enlargement at early stages of diabetes in male rats with T2DM (Tsounapi et al. 2019). We found increased nitrotyrosine levels in the bladders of 9-week diabetic mice (Elrashidy et al. 2019), 20-week (Xiao et al. 2013), and 44-week (Elrashidy and Liu 2019) diabetic rats. Increased lipid peroxidation products and aldose reductase were found to be associated with the decrease in detrusor muscle contractility in alloxan-induced diabetic rabbits (Changolkar et al. 2005). Increased oxidative stress can damage smooth muscle cells (Kanika et al. 2011), interrupt neurotrophins necessary for neuron survival (Whitmire et al. 2011), and induce apoptosis (Ha et al. 2013; Chen et al. 2015; Lin et al. 2020), which could contribute to diabetic cystopathy. Several antioxidant agents were studied on diabetic bladder dysfunction. The cyanidin-3-O-β-D-glucopyranoside fraction of mulberry fruit treatment in STZ-diabetic male rats has markedly ameliorated bladder capacity, maximal detrusor pressure, contraction frequency, and contaction interval through reducing oxidative stress and apoptosis (Ha et al. 2013). Similarly, grape seed proanthocyanidin extract as a preventive treatment was found to decrease oxidative stress markers and apoptosis in STZ-induced diabetic female rat bladder; a beneficial effect of the extract in mitigating functional and histological changes of bladder was attributed to activation of antioxidant nuclear erythroid related factor2 (Nrf2) mediated pathway (Chen et al. 2015). Supporting the role of Nrf2 in pathogenesis of diabetic bladder, prophylactic antioxidant treatment with the Nrf2 activator sulforaphane remarkably reduced bladder capacity and mictirituon duration by reducing reactive oxygen species (ROS) levels and related endoplasmic reticulum stress and apoptosis (Lin et al. 2020). Increased intracellular and mitochondrial ROS levels were accompanied by increased apoptosis of bladder smooth muscle cells in 11-week STZ-induced diabetic female rats. IR-61, is a fluorescent dye with antioxidant effect, treatment (1.6 mg/kg/week, i.p.) for 10-week markedly reduced oxidative stress and apoptosis in bladder tissue and improved the bladder dyfunction compared to diabetic rats (Wang et al. 2021). Considering the role of oxidative stress in pathogenesis of diabetic bladder and beneficial effects of antioxidant agents, it is reasonable to target oxidative stress to treat bladder complications in diabetes.
Inflammation
Inflammation may play a role in the pathophysiology of diabetic bladder dysfunction as in other complication of diabetes (Navarro and Mora 2005). The role of several inflammatory mediators in diabetic bladder dysfunction has been studied. Elevated tumor necrosis factor-α (TNF-α) levels in serum and bladder tissue were shown in a genetic T2DM model, DKO female mice. TNF-α was shown to be implicated in bladder dysfunction exhibiting as overactivity in early (12-week) stage and hypoactivity in late (20-week) stages of diabetes. Six-week-old animals were administered a TNFRI (inhibitor of TNF-α) (2 mg/kg, twice per week, i.p.) for 6 weeks, and frequency of nonvoiding contractions, voided volume, and micturition volume were improved in treated mice compared to diabetic group. Moreover, concomitant administration of TNFRI and metformin yielded a better outcome in reducing frequency of nonvoiding contractions compared to TNFRI alone (Wang et al. 2012). TLR4, an innate immune receptor, was upregulated 4 weeks after STZ injection in male mice. Elevated inflammatory and oxidative stress response due to increased TLR4 activation was found to mediate bladder hypertrophy and increased bladder contractility in diabetes. These changes were attenuated in TLR4 knockout diabetic mice compared to wild-type diabetic group (Szasz et al. 2016). The NLRP3 inflammasome was found to be activated in T1DM Akita female mice. NLRP3-mediated inflammation was implicated in decreased sense of bladder, void volume, and voiding efficiency as well as increased increased frequency of voiding and postvoid residual volume in diabetic mice compared to control. Depletion of NLPR3 gene prevented the unfavorable effect of diabetes on bladder (Hughes et al. 2019). Botulinum toxin A was approved by FDA and NICE in OAB patients who do not respond or tolerate anticholinergic drugs (Ibrahim et al. 2022). Intravesical injection of botulinum toxin A inhibits both hypersensivity of detrusor muscle and chronic inflammation. Thereafter, it has been used successfully in the treatment of diabetes-related OAB in patients, yet more studies are needed to ensure efficacy and safety of botulinum toxin A (Wang et al. 2020a). Considering the possible role of inflammation in diabetic bladder dysfunction, targeting inflammatory pathways might be beneficial to treat complications.
Rac1
The GTPase Rac1 has a role in the oxidative stress response and also is essential for the contraction of the smooth muscle (Rahman et al. 2014). An augmented Rac1 expression and an increased retinal ROS production were found to be involved in the development of diabetic retinopathy (Mohammad et al. 2019). The expression of Rac1 was found to be increased in overall bladder of STZ-induced diabetes in rats (Laddha and Kulkarni 2022), in bladder smooth muscle, but not urothelium in mice with STZ-induced diabetes (Poladia and Bauer 2004). Another study using STZ-treated rats found that Rac1 immunoreactivity was increased in the epithelium, lamina, propria and tunica muscularis of the bladder; this was attenuated but not eliminated by insulin treatment (Evcim et al. 2015). In isolated bladder strips obtained 8 or 12 weeks after the STZ injection, the Rac1 inhibitor NSC23766 (0.1, 1, and 10 µM) attenuated carbachol-induced contraction in all groups, but the extent of inhibition was greater in the diabetic and insulin-treated than the nondiabetic rats (Evcim et al. 2015). While these data leave it open whether Rac1 is solely increased in smooth muscle or also in mucosal cells, they indicate that the role of Rac1 may specifically increase in diabetes and make Rac1 a potential target for diabetic uropathy treatment. Accordingly, inhibitors of Rac1 such as EHT1864 or NSC23766 can attenuate contraction elicited via multiple receptors in the human prostate (Wang et al. 2015) and bladder (Li et al. 2020). Moreover, inhibition of Rac1 by small molecules or by gene silencing reduced growth and actin organization of human bladder smooth muscle cells (Wang et al. 2020b). A limitation of these findings is that NSC23766 is not only a direct inhibitor of Rac1 but also a competitive antagonist of muscarinic receptors (Levay et al. 2013). This complication may be absent with EHT1864 (Li et al. 2020). Another complication is that both NSC23766 and EHT1864 showed considerable effects in Rac1 knockout cells, implying that they have Rac1-independent effects (Wang et al. 2020b). Nonetheless, the overall data suggest that Rac1 may play a role both in contraction and hypertrophy development in the LUT. Treatment with daidzein dose-dependently attenuated the increased Rac1 expression in diabetic rats (Laddha and Kulkarni 2022).
Nerve growth factor
Nerve growth factor (NGF) and its receptors including p75 are expressed in urinary bladder and have an essential role in its innervation and maintenance of function (Ochodnicky et al. 2011; Elrashidy and Liu 2019). While several studies have explored the expression of NGF and p75 in the bladder of STZ-injected rats, the reported findings are inconclusive: several studies reported a reduced expression (Sasaki et al. 2002; Tong and Cheng 2005; Jiang et al. 2008; Nirmal et al. 2014; Elrashidy and Liu 2019), but some also reported at least transient increases (Koo et al. 1993; Steinbacher and Nadelhaft 1998). If a potential upregulation is transient as proposed by some (Koo et al. 1993), studies with few time points may have missed that. This also makes it difficult to interpret single timepoint data from other models such as fructose-fed rats (Cheng and Tong 2008). Nonetheless, these findings motivated several investigators to explore effects of interventions on bladder function and the NGF system or those directly targeting NGF. Treatment with either insulin or the sodium-glucose transporter 1 and 2 inhibitor phlorizin prevented the STZ-induced reduced expression of NGF and its receptor, p75 (Tong and Cheng 2005). Treatment with α-lipoic acid starting 6 weeks after STZ injection normalized the lowered NGF expression and bladder function (Jiang et al. 2008). After a feasibility study had demonstrated successful NGF expression in mice using a Herpes simplex virus (Goins et al. 2001), the same group reported that injection of this vector into the bladder wall 8 weeks after STZ injection restored lower NGF levels and the impaired bladder function as assessed by cystometry (Sasaki et al. 2004). Another approach to increase NGF levels has been the use of human umbilical cord mesenchymal stem cells transduced with the NGF gene in STZ diabetic rats (WenBo et al. 2017). When administered 3 days after the STZ injection, the impaired voiding function was improved. Unlike NGF, proNGF (a precursor of NGF) induces inflammation and degenerative pathways although both use the p75 receptor. In a mouse STZ model, an proNGF antibody or a small molecule p75 inhibitor were tested (Mossa et al. 2020). The p75 antagonist reduced STZ-induced bladder enlargement, whereas the proNGF antibody did not. On the other hand, the antibody normalized impaired bladder function, whereas the antagonist did not. The authors speculated that it may be less NGF levels but rather the proNGF/NGF ratio that is important for amelioration of diabetes-associated bladder dysfunction.
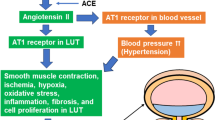
Angiotensin II type 1 receptors
Angiotensin II plays a role in the pathophysiology of many diseases, and inhibitors of the angiotensin II type 1 (AT1) receptors are effective in various human diseases and in animal models of even more conditions (Michel et al. 2016). Specifically, AT1 antagonists exert antihypertrophic effects on various tissues, particularly related to smooth muscle hypertrophy. The best studied model of bladder hypertrophy is surgically induced bladder outlet obstruction, most often applied to rats. The AT1 antagonist telmisartan was reported to attenuate, but not abolish bladder enlargement in this model, whereas the obstruction-associated decrease in AT1 receptor binding was abolished (Yamada et al. 2009). Another study did not confirm the attenuation of bladder hypertrophy by telmisartan but found that telmisartan abolished the obstruction-induced increase in AT1 receptors and NGF in both detrusor and urothelium (Cho et al. 2012). In cystometric investigations, telmisartan attenuated the shortening of the inter-contraction intervals and abolished the increase in nonvoiding bladder contractions. Interestingly, the combined presence of bladder outlet obstruction and STZ-induced diabetes caused greater bladder enlargement and disturbance of contractile function than either condition alone in one study (Longhurst et al. 2004), but this was not confirmed in a later study by other investigators (Öztürk et al. 2002). Based on these findings, the AT1 antagonist valsartan (starting 2 weeks after STZ) was tested in the rats exposed to a combination of low-dose STZ and a HFD, a model of type 2 diabetes (Arioglu Inan et al. 2022). However, valsartan did not reverse bladder enlargement, nor did it affect contractile or relaxant response in isolated bladder strips. Thus, the limited data on AT1 antagonist are inconsistent within the obstruction model and in comparison, with diabetes.
Serotonin receptors
Autonomic neuropathy in diabetes leads to reduced sense of bladder filling and is associated with diabetes-related bladder dysfunction (Duby et al. 2004). Central serotonin (5-HT) receptors are localized in nerve terminals and have variable effects (mostly inhibitory) in the control of micturition depending on receptor subtype and species (Ramage 2006). STZ-induced T1DM for 8 weeks in female rats resulted in reduced voiding function accompanied with increased bladder capacity and residual volume of urine and impaired external urethral sphincter activity. Cumulative dose of 8-OH-DPAT (0.003–1 mg/kg, i.v.), a selective 5-HT1A agonist, was administered during filling cystometry study. 8-OH-DPAT improved the voiding efficacy through increasing micturition volume and urethral sphincter activity as well as decreasing bladder capacity, residual volume, and peak bladder pressure in diabetic rats (Gu et al. 2012). In a subsequent study by same study group, the effect of (2,5-dimethoxy-4-idophenyl)-2-aminopropane hydrochloride (DOI), 5-HT)2A/2C receptor agonist was evaluated in similar experimental setting. Cumulative administration of (0.01–0.3 mg/kg, i.v.) improved voiding efficacy by increasing micturition volume and high-frequency oscillation activity and by decreasing bladder capacity, peak bladder pressure, residual volume, and contraction duration in diabetic rats (Tu et al. 2015). Targeting 5-HT receptors seem reasonable in the treatment of LUTD in diabetes.
Adenosine receptors
A beneficial effect of the adenosine receptor antagonist caffeine on voiding dysfunction in diabetes was shown by a research group from China with several studies. Caffeine (10 mg/kg/day, p.o.) and coffee (caffeine dose 286 mg/kg/day, p.o.) treatments were started at the second day of STZ-induced diabetes and performed for 7 weeks in male rats. These preventive approaches decreased bladder weight, treshold volume for micturition, residual volume, and bladder capacity compared to diabetic rats. Impaired neurogenic and acetylcholine-mediated contraction of bladder strips due to diabetes were subtantially rectified in treated rats. Increased cAMP levels in bladder with both coffee and caffeine are another finding that was ascribed to improved bladder function (Yi et al. 2006). The effect of caffeine treatment in STZ-induced diabetic bladder was evaluated at different doses (5 mg/kg/day and 10 mg/kg/day, p.o.) for 8 weeks in female rats. Caffeine treatment markedly reduced bladder weight, bladder capacity, voiding time, peak voiding pressure, and residual urine volume compared to diabetic rats, and these effects were comparable at both doses. Peak voiding pressure and voiding efficacy were significantly improved in rats treated with both doses of caffeine, yet the effect was greater at 10 mg/kg/day caffeine (Liu et al. 2019). In a further study, female STZ-diabetic rats were treated with caffeine (10 mg/kg/day) for 16 weeks (Xue et al. 2021). Caffeine treatment was found to improve voiding dysfunction by increasing expression of NGF, brain-derived neurotrophic factor, and calcitonin gene-related peptide in bladder tissue and by reducing apoptotic cells in dorsal root ganglion. Reduced antiapoptotic protein Bcl-2 level in diabetic rats was markedly increased with caffeine treatment, and caffeine decreased the level of pro-apoptotic proteins such as Bax, cleaved caspase-3, and cleaved caspase-9 in dorsal root ganglion (Xue et al. 2021).
Soluble guanylyl cyclase
Soluble guanylyl cyclase (sGC) is an enzyme which is activated by nitric oxide (NO). Activation of sGC increases cellular cGMP levels and mediates tissue specific responses. cGMP mediates relaxation in bladder and other smooth muscle preparations. Thus, the NO-sGC-cGMP pathway may be important in the treatment of voiding and storage disorders (Rahnama'i et al. 2013). HFD-fed obese mice had increased voiding frequencies, nonvoiding bladder contractions, and receptor-dependent (induced by carbachol) and receptor-independent (induced by KCl and CaCl2) bladder contractile responses (Leiria et al. 2014). These results were accompanied by a decreased expression of sGC α1 and β1 subunits and cGMP levels in bladder tissue. Oral administration of 1 mg/kg BAY 60–2770 (an sGC activator) to obese mice for 2 weeks fully reversed the above changes in the bladder (Leiria et al. 2014). In another study, the mRNA expression of PDE5 was increased, while cGMP levels were lower in the urethra in STZ-induced 8-week T1DM rats than those in the control group (Gotoh et al. 2021). Urethral pressure reduction and high-frequency oscillation amplitude during voiding were decreased in T1DM rats, indicating impaired urethral relaxation. sGC activation (BAY 60–2770, 1 mg/kg/day for 2 weeks, oral) reversed the changes of PDE5 expression and cGMP levels and improved cystometric (opening pressure, closing pressure and % voiding efficiency) and urethra perfusion parameters (urethral pressure at the beginning of relaxation, urethral pressure reduction and high frequency oscillation amplitude) in diabetic animals (Gotoh et al. 2021). These studies demonstrated that activation of sGC is an effective option for the treatment LUTD in DM. However, more studies are needed to clarify their effect and efficacy in diabetes related LUT dysfunction.
Myoinositol
Myoinositol, the most common isomeric form of inositol, is naturally found in plant and animal cells and also is produced from glucose in human cells. Myoinositol has various beneficial effects in diabetes including improvement of insulin sensitivity by showing an insulin-like effect (Croze and Soulage 2013). In addition to its insulin sensitizing effect, myoinositol has a beneficial effect on hyperlipidemia which is a common feature of type 2 diabetes (Antony et al. 2017). Myoinositol was shown to increase insulin sensitivity through upregulation of peroxisome proliferator-activated receptor γ and the glucose transporter GLUT4 in adipose tissue of HFD + STZ-induced T2DM rats (Antony et al. 2017). On the other hand, accumulation of sorbitol in cells due to hyperglycemia subsequently leads to decreased myoinositol levels in diabetes. There are limited studies related to the effect of myoinositol on LUT dysfunction due to diabetes. STZ diabetic rats received 1 g/kg/day of myoinositol via drinking water for 8 weeks beginning 3 days after STZ injection (Gousse et al. 1991). Myoinositol treatment did not restore blood glucose levels, reduced prostate size, or reduced prostate β-adrenoceptor density compared to diabetic animals. A study of similar design investigated the effect of myoinositol on muscarinic receptors in the prostate. Diabetes resulted in reduced prostate size and downregulation in prostate M3 receptor level, and myoinositol treatment did not improve either parameter (Latifpour et al. 1991). In a subsequent study by the same group, myoinositol showed no effect on increased bladder size and increased bladder muscarinic receptor levels in STZ induced diabetic rats (Latifpour et al. 1992).
Daidzein
Daidzein is an isoflavone mostly found in soybean, legumes, and soy-based products. It also known as a phytoestrogen due to its similar chemical structure with mammalian estrogen (Alshehri et al. 2021). Estrogen receptors were shown to mediate cytoprotective effects of daidzein in vitro (Xu et al. 2009; Robb and Stuart 2014). The effect of daidzein in diabetes and in diabetes-related complications gained attention in recent years. Daidzein exerted beneficial effects on glucose and lipid homeostasis in T2DM mice models (Park et al. 2006; Cheong et al. 2014). Daidzein prevented onset of diabetes in nonobese diabetic (NOD) mice (Choi et al. 2008) and promoted significant decrease in postprandial glucose levels in STZ-induced diabetic mice (Park and Ju 2013). Daidzein induced PPARγ activation which leads enhanced insulin sensitivity and glucose uptake in murine 3T3-L1 adipocyte cells (Cho et al. 2010). Anti-inflamatory and/or antioxidant effects of daidzein were shown in diabetes’ several related complications such as endothelial dysfunction, neuropatic pain, retinopathy, and cardiomyopathy (Roghani et al. 2013; Jia et al. 2020; Laddha and Kulkarni 2021a, 2021b). Besides, daidzein showed antitumorigenic activity against bladder carcinoma (Su et al. 2000; He et al. 2016). Urinary bladders from Wistar male rats subjected to in vitro anoxia-glucopenia and subsequent reperfusion. Incubation with daidzein, 60-min before anoxia-glucopenia, during anoxia-glucopenia, and first 30-min of reperfusion, resulted in reduced electrical field stimulation-induced contraction concentration dependently. In the same study, rats were received daidzein treatment (2 or 20 mg/kg/day, s.c.) for 1 week. At the end of treatment, bladder tissue exposed to anoxia-glucopenia and reperfusion. Electrical field stimulation-induced contraction responses in low-dose daidzein-treated rats were found significantly higher. Moreover, malondialdehyde generation in treated rats were found lower. Combining the direct relaxant effect of daidzein against electrical field stimulation-induced contraction in detrusor strips, with neuroprotective and antioxidant effect of the compound, may be potential treatment strategy in LUT dysfunction (Valeri et al. 2012). Daidzein was shown to be a promising treatment agent against the complications of diabetes in several studies. However, little is known on diabetic LUT function. Considering its beneficial effects in diabetes and on bladder, this venue is worth to be elucidated. Recent work indicates that treatment with daidzein in the rat STZ model of diabetes prevented the loss of antioxidant enzyme activity and attenuated the elevated expression of NADP oxidase and Rac1 in the bladder; concomitantly, it improved bladder capacity, voiding efficiency, and residual urine in cystometric studies and improved mucosal lymphocyte infiltration and multifocal hyperplasia of the transitional epithelium (Laddha and Kulkarni 2022).
Nonpharmacological interventions
Some patients may not benefit adequately from established pharmacological treatment approaches used in diabetes-related LUTS. Therefore, nonpharmacological interventions could be a choice of therapy in these patients.
Stem cell therapy
Stem cell-based therapy approaches have gained popularity in recent years to treat a variety of diseases including untreatable disorders (Zakrzewski et al. 2019). Different types of stem cells were evaluated in diabetic bladder dysfunction. Adipose-derived stem cells was injected either into the tail vein (DM + T group) or into the detrusor (DM + B group), 3-month after HFD + low-dose STZ diabetes induction in female rats. One month after treatment, bladder function was evaluated. Reduced micturition interval and urine volume per void were improved voiding function which was increased by 40% in DM + T group and by 60% in DM + B group compared to diabetic group. Adipose-derived stem cells promoted angiogenesis and inhibited apoptosis in vivo and in vitro, which were postulated to be the underlying mechanisms of its beneficial effect on diabetic bladder function (Zhang et al. 2012). Human embryonic stem cell-derived multipotent mesenchymal stem cells (M-MSCs) at three different doses (0.25, 0.5, and 1 × 106 cells) were transplated to STZ-induced diabetic female rats 3 weeks after diabetes induction. Bladder function was assessed 1, 2, and 4 weeks after M-MSCs transplantation. Bladder underactivity that worsened time dependently was observed in diabetic animals. M-MSCs improved contractile function and voiding efficiency of bladder. Myogenic activity of bladder was restored through integration of M-MSCs into pericytes. M-MSC treatment was rectified increased fibrosis, apoptosis and mast cell infiltration due to diabetes as well (Shin et al. 2020). In another study, STZ-diabetic male rats were administered integrin-linked kinase gene-modified bone marrow-derived stem cells intravenously at 2-week after diabetes induction. Four weeks after, abnormalities in bladder function was improved with gene-modified stem cell treatment. A beneficial effect of integrin-linked kinase gene-modified stem cells was attributed to reduced apoptosis and increased proliferation, migration and angiogenesis via distinct molecular pathways (Huang et al. 2020). Besides the well-known types of MSCs, the effect of a new type of MSCs, neonatal bladder-derived mesenchymal stem cells (nMSC-B), was evaluated in diabetic bladder. nMSC-B was administered either i.p. or directly to the bladder wall to 12-week STZ diabetic male rats and 4 weeks after application bladder function was evaluated. nMSC-B were found to be effective to restore bladder function (Boga et al. 2020). Beneficial effects of different stem cell therapies in diabetic bladder dysfunction ascribed to its paracrine effect (Zhang et al. 2012; Huang et al. 2020; Shin et al. 2020). Stem cell therapy stands as a promising approach in diabetic bladder treatment that needs to be supported by further clinical tirals.
Low-intensity extracorporeal shockwave therapy (Li-ESWT)
Li-ESWT is a noninvasive method which has been shown to be a promising treatment approach in several diseases including urological disorders (Lin et al. 2021). Recently, the effect of Li-ESWT in the treatment of bladder dysfunction was invastigated in different studies. Li-ESWT was applied once weekly for 4 weeks to STZ-diabetic female rats 4-week after diabetes induction. Cystometry analysis was revealed increased postvoid residual volume, voided volume, micturition interval and decreased maximal detrusor pressure due to diabetes, and Li-ESWT ameliorated postvoid residual volume and maximal detrusor pressure to some extent. Contractile function of bladder was abolished in response to electrical-field and muscarinic receptor-mediated stimulation in diabetic animals, Li-ESWT restored the contractile activity. Additionally, morphological and neurological abnormalities in bladder were abundantly improved by Li-ESWT (Wang et al. 2018). In another study, Li-ESWT was given to HFD (4-week) + low-dose STZ diabetic female rats 1-week after STZ injection, twice a week for 4-week. Li-ESWT was markedly improved micturition interval and maximal detrusor pressure which were reduced due to diabetes. Besides improving voiding function, Li-ESWT was restored nerve expression and was activated angiogenesis in bladder tissue (Lee et al. 2021). Beneficial effect of Li-ESWT in diabetic bladder was attributed to reduced gene expression of transient receptor potential vanilloid 1, interleukin-1β, the muscarinic receptors M2 which are related to mechanosensation, ischemia/inflammation and bladder contraction respectively. Li-ESWT did not affect other muscarinic receptor subtype M1 and M3 expression (Dimitriadis et al. 2021). Li-ESWT may be a promising approach to restore bladder function in diabetic patients.
Neuoromodulation
As previously stated, neuropathy is one of the underlying causes of diabetes related bladder dysfunction. Neuromodulation was shown to restore voiding function in several studies (Chen et al. 2013; Hsieh et al. 2016; Han et al. 2021). Voiding efficacy in control animals was found about 75% while it was measured as 30.02 ± 7.89% after 8 weeks of diabetes and 19.98 ± 6.03% after 18 weeks of diabetes in STZ-induced T1DM male rats (Chen et al. 2013). These data shows that time-dependent reduction of bladder function due to diabetes. Besides reduced voiding efficiency, diabetic animals showed increased volume treshold, contraction amplitude, residual volume, bursting period and had decreased intercontraction interval, voided volume. Acute electrical stimulation of pudendal sensory nerve at low intensity (0.025–0.05 mA) resulted in an increase in voiding efficacy only in 8-week diabetic animals. When, intensity of electrical stimulation was elevated to 0.1–0.3 mA, voiding fuction was restored in both 8-week and 18-week diabetic animals by reduced residual volume and increased intercontraction interval, contraction duration and bursting period. Following 0.1–0.3 mA electrical stimulation voiding efficiency was markedly increased to 40–50% but was not reach to control levels (Chen et al. 2013). In another study, 4 days after induction of diabetes with STZ injection, female rats were subjected to chronic electrical stimulation (3 or 6 weeks) (10 µA) of pudendal sensory nerves . Voiding efficiency in control rats was about 64%, which decreased to 45% and 17% in 3 weeks and 6 weeks of diabetes, respectively. Chronic electrical stimulation therapy resulted in better voiding function, which was increased from 45 to 57% with 3 weeks and from 17 to 51% with 6-week of application. Electrical stimulation improved abnormalities in bladder function parameters such as contraction duration, residual volume, bursting period independent of its duration. Axonal desity of pudendal nerve was markedly decreased in 6-week diabetic animals which was reversed by chronic electirical stimulation (Hsieh et al. 2016). Electroacupuncture treatment for 8 weeks was eveluated in a T2DM model induced by combination of HFD (4-week) + STZ injection. At the end of 12 weeks, diabetic animals presented with bladder hypertrophy, increased residual volume and volume threshold for micturition. All of the abnormalities were improved by electroacupuncture therapy and its beneficial effect was ascribed to regulation of myosin light chain and myosin light chain kinase phosphorylation. Moreover, electroacupuncture was reduced infiltration of inflammatory cells in bladder (Han et al. 2021). These studies suggests that electrical stimulation of bladder may prevent or improve bladder dysfunction in diabetic individuals by facilitating bladder contraction.
Conclusions
The prevalence of diabetes and hence its undesirable effects increases continuously and affects millions of people worldwide. Diabetes-related macrovascular and microvascular diseases and treatment approaches are widely studied due to their high morbidity and mortality rate. Nevertheless, LUTD related to diabetes is a very common complication that considerably impairs quality of life. Thus, efficient treatment approaches are needed for diabetic people who suffers from LUTD. Considering the mainstay LUTD medications in diabetes, α1-adrenoceptor antagonists have comparable effect in clinical studies in diabetic and nondiabetic patients, whereas preclinical studies show inconsistent findings. Based on few studies, 5α-reductase inhibitors may worsen the diabetes-associated LUTD. In preclinical studies, the PDE5 inhibitor tadalafil was shown to have beneficial effect against LUTD in diabetes. Muscarinic receptor antagonists were found to be a little less effective in the treatment of OAB with concomitant diabetes in preclinical and clinical studies, however further assessment is needed for conclusion. The effect of β3-adrenoceptor agonists on LUT function in diabetes setting need to be studied.
There are few clinical studies investigating the possible effects of antidiabetic treatment agents on LUT dysfunction in T1DM and to the best our knowledge, there are no studies in T2DM. Moreover, most of the preclinical studies have been conducted on animal models of T1DM. Considering the high incidence of T2DM over T1DM, findings from preclinical studies remain insufficient for clinical translation. Insulin was shown mostly to prevent diabetes-associated LUT dysfunction as well as can reverse the symptoms. Metformin showed some beneficial effects on LUTD in normoglycemic animals. Effect of other T2DM medications (SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, GLP-1 agonists) on LUTD in diabetes remains to be studied. Based on our search, there might be possible novel pathways involved in the development of LUTD in diabetes and promising candidate compounds in the treatment of diabetes-associated LUT dysfunction. In preclinical studies involvement of oxidative stress, inflammation, GTPase Rac 1, NGF, angiotensin II, 5-HT and adenosine receptors and sGC in LUT function have been demonstrated in normoglycemic and/or hyperglycemic conditions. Hence, compounds targeting these pathways may be beneficial in the treatment of diabetic LUTD. However, data on diabetes related LUTD either are few and inconsistent or do not exist. Thus, more studies are needed with these compounds. Other than pharmacological treatments, nonpharmacological interventions such as stem cell therapy, Li-ESWT and neuoromodulation were found to be effective in diabetic bladder treatment in preclinical studies. These approaches may be a promising alternative for those patients who can not benefit from standart trerapy regimen.
Our selective literature search found an insufficient number of preclinical and clinical studies on either LUTD medications in diabetes or antidiabetic treatments on LUTD to derive robust conclusion. Inconsistent data or low clinical relevance due to study structure make interpretation of the data difficult. There are points to be elucidated in this area by further research. Hence, properly conducted preclinical and clinical studies are required to meet the need in this field.
Abbreviations
- BPH:
-
Benign prostatic hyperplasia
- DM:
-
Diabetes mellitus
- GLP-1:
-
Glucagon-like peptide 1
- HFD:
-
High fat diet
- IPSS:
-
International Prostate Symptom Score
- Li-ESWT:
-
Low-intensity extracorporeal shockwave therapy
- LUT:
-
Lower urinary tract
- LUTD:
-
Lower urinary tract dysfunction
- LUTS:
-
Lower urinary tract symptoms
- M-MSC:
-
Multipotent mesenchymal stem cells
- nMSC-B:
-
neonatal bladder-derived mesenchymal stem cells
- NGF:
-
nerve growth factor
- NO:
-
Nitric oxide
- OAB:
-
Overactive bladder syndrome
- PDE5:
-
Phosphodiesterase type 5
- ROS:
-
Reactive oxygen species
- SGLT2:
-
Sodium-glucose transporter 2
- sGC:
-
Soluble guanylyl cyclase
- STZ:
-
Streptozotocin
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- TNF-α:
-
Tumor necrosis factor-α
- UAB:
-
Underactive bladder syndrome
References
Alshehri MM, Sharifi-Rad J, Herrera-Bravo J, Jara EL, Salazar LA, Kregiel D, Uprety Y, Akram M, Iqbal M, Martorell M (2021) Therapeutic potential of isoflavones with an emphasis on daidzein. Oxidative Med Cell Longev 2021:6331630. https://doi.org/10.1155/2021/6331630
Antony PJ, Gandhi GR, Stalin A, Balakrishna K, Toppo E, Sivasankaran K, Ignacimuthu S, Al-Dhabi NA (2017) Myoinositol ameliorates high-fat diet and streptozotocin-induced diabetes in rats through promoting insulin receptor signaling. Biomed Pharmacother 88:1098–1113. https://doi.org/10.1016/j.biopha.2017.01.170
Antunes-Lopes T, Vasconcelos A, Costa D, Charrua A, Neves J, Silva J, Cruz F, Silva C (2019) The impact of chronic pelvic ischemia on LUTS and urinary levels of neuroinflammatory, inflammatory, and oxidative stress markers in elderly men: a case-control study. Urology 123:230–234. https://doi.org/10.1016/j.urology.2018.09.004
Arioglu Inan E, Ellenbroek JH, Michel MC (2018) A systematic review of urinary bladder hypertrophy in experimental diabetes: part I. streptozotocin-induced rat models. Neurourol Urodyn 37:1212–1219. https://doi.org/10.1002/nau.23490
Arioglu Inan E, Bese OD, Karaca GE, San HS, Kayki Mutlu G, Erdogan BR, Michel MC (2022) The angiotensin II receptor antagonist valsartan does not attenuate diabetes-induced bladder enlargement in rats. Neurourol Urodyn Press
Atalay HA, Akarsu M, Canat L, Ülker V, Alkan İ, Ozkuvancı U (2017) Impact of poor glycemic control of type 2 diabetes mellitus on serum prostate-specific antigen concentrations in men. Prostate Int 5:104–109. https://doi.org/10.1016/j.prnil.2017.02.004
Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker-Corkery E, Lepor H (1995) Benign prostatic hyperplasia specific healthy status measures in clinical research: how much change in the American Urological Association Symptom Index and the Benign Prostatic Hyerplasia Impact Index is perceptible to patients? J Urol 154:1770–1774. https://doi.org/10.1016/S0022-5347(01)66780-6
Beckman JA, Creager MA (2016) Vascular complications of diabetes. Circ Res 118:1771–1785. https://doi.org/10.1161/circresaha.115.306884
Boga MS, Haliloğlu AH, Gülpınar Ö, Özayar A, Sönmez MG, Pinarli FA, Boğa E, Pinarci T, Tiryaki M, Göğüş O (2020) Neonatal bladder-derived mesenchymal stem cells ameliorate bladder dysfunction in diabetic rat models. Urol J 17:413–421. https://doi.org/10.22037/uj.v0i0.5504
Bozlu M, Ulusoy E, Cayan S, Akbay E, Görür S, Akbay E (2004) A comparison of four different alpha1-blockers in benign prostatic hyperplasia patients with and without diabetes. Scand J Urol Nephrol 38:391–395. https://doi.org/10.1080/00365590410015678
Bubb KJ, Ravindran D, Cartland SP, Finemore M, Clayton ZE, Tsang M, Tang O, Kavurma MM, Patel S, Figtree GA (2021) β3-Adrenergic receptor stimulation promotes reperfusion in ischemic limbs in a murine diabetic model. Front Pharmacol 12:666334. https://doi.org/10.3389/fphar.2021.666334
Changolkar AK, Hypolite JA, Disanto M, Oates PJ, Wein AJ, Chacko S (2005) Diabetes induced decrease in detrusor smooth muscle force is associated with oxidative stress and overactivity of aldose reductase. J Urol 173:309–313. https://doi.org/10.1097/01.ju.0000141583.31183.7a
Chen SC, Lai CH, Fan WJ, Peng CW (2013) Pudendal neuromodulation improves voiding efficiency in diabetic rats. Neurourol Urodyn 32:293–300. https://doi.org/10.1002/nau.22280
Chen S, Zhu Y, Feng X, Zhang Z, Li S, Shi B (2014) Changes in alpha1-adrenoceptor and NGF/proNGF pathway: a possible mechanism in diabetic urethral dysfunction. Urol Int 93:344–351. https://doi.org/10.1159/000355711
Chen S, Zhu Y, Liu Z, Gao Z, Li B, Zhang D, Zhang Z, Jiang X, Liu Z, Meng L, Yang Y, Shi B (2015) Grape seed proanthocyanidin extract ameliorates diabetic bladder dysfunction via the activation of the Nrf2 pathway. PLoS ONE 10:e0126457. https://doi.org/10.1371/journal.pone.0126457
Chen L, Lv L, Zhang L, Gao Z, Liu Y, Wang S, Zhou N, Xia Y, Cui J, Jiang X, Zhang X, Li Y, Shi B (2021) Metformin ameliorates bladder dysfunction in a rat model of partial bladder outlet obstruction. Am J Physiol Renal Physiol 320:F838-f858. https://doi.org/10.1152/ajprenal.00625.2020
Cheng JT, Tong YC (2008) Alterations of nerve-growth factor and p75 NTR expression in urinary bladder of fructose-fed obese rats. Neurosci Lett 441:25–28. https://doi.org/10.1016/j.neulet.2008.06.008
Cheong SH, Furuhashi K, Ito K, Nagaoka M, Yonezawa T, Miura Y, Yagasaki K (2014) Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in Type 2 diabetic model mice. J Nutr Biochem 25:136–143
Cho KW, Lee O-H, Banz WJ, Moustaid-Moussa N, Shay NF, Kim Y-C (2010) Daidzein and the daidzein metabolite, equol, enhance adipocyte differentiation and PPARγ transcriptional activity. J Nutr Biochem 21:841–847
Cho ST, Park EY, Kim JC (2012) Effect of angiotensin II receptor antagonist telmisartan on detrusor overactivity in rats with bladder outlet obstruction. Urology 80:1163e1111-1163e1167. https://doi.org/10.1016/j.urology.2012.05.002
Choi M, Jung U, Yeo J, Kim M, Lee M (2008) Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab Res Rev 24:74–81
Choi H, Bae JH, Oh CY, Jeong SJ, Ko WJ, Choi JB, Seo JT, Lee DH, Kim JC, Lee KW, Kim YH (2018) Clnical efficacy of solifenacin in the management of diabetes mellitus-associated versus idiopathic overactive bladder symptoms: a multicenter prospective study. Int Neurourol J 22:51–57. https://doi.org/10.5213/inj.1834982.491
Christ GJ, Hsieh Y, Zhao W, Schenk G, Venkateswarlu K, Wang HZ, Tar MT, Melman A (2006) Effects of streptozotocin-induced diabetes on bladder and erectile (dys)function in the same rat in vivo. BJU Int 97:1076–1082. https://doi.org/10.1111/j.1464-410X.2006.06058.x
Croze ML, Soulage CO (2013) Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 95:1811–1827. https://doi.org/10.1016/j.biochi.2013.05.011
Cypess AM, Weiner LS, Roberts-Toler C, Franquet E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, Kolodny GM (2015) Activation of human brown adipose tissue by a ß3-adrenergic receptor agonist. Cell Metab 21:33–38. https://doi.org/10.1016/j.cmet.2014.12.009
Daneshgari F, Moore C (2006) Diabetic uropathy. Semin Nephrol 26:182–185. https://doi.org/10.1016/j.semnephrol.2005.09.009
Daneshgari F, Huang X, Liu G, Bena J, Saffore L, Powell CT (2006) Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice. Am J Physiol Regul Integr Comp Physiol 290:R1728-1735. https://doi.org/10.1152/ajpregu.00654.2005
Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, Chacko S (2009) Diabetic bladder dysfunction: current translational knowledge. J Urol 182 Suppl.: S18-S26. https://doi.org/10.1016/j.juro.2009.08.070
Dimitriadis F, Papaioannou M, Sokolakis I, Fragou A, Hatzichristou D, Apostolidis A (2021) The effect of low-intensity extracorporeal shockwave treatment on the urinary bladder in an experimental diabetic rat model. Int Neurourol J 25:34–41. https://doi.org/10.5213/inj.2040344.172
Duby JJ, Campbell RK, Setter SM, White JR, Rasmussen KA (2004) Diabetic neuropathy: an intensive review. Am J Health Syst Pharm 61:160–173
Elabbady A, Hashad MM, Kotb AF, Ghanem AE (2016) Studying the effect of type 2 diabetes mellitus on prostate-related parameters: A prospective single institutional study. Prostate Int 4:156–159. https://doi.org/10.1016/j.prnil.2016.07.005
Ellenbroek JH, Arioglu Inan E, Michel MC (2018) A systematic review of urinary bladder hypertrophy in experimental diabetes: Part 2. Comparison of animal models and functional consequences. Neurourol Urodyn 37:2346–2360. https://doi.org/10.1002/nau.23786
Elrashidy RA, Liu G (2019) Long-term diabetes causes molecular alterations related to fibrosis and apoptosis in rat urinary bladder. Exp Mol Pathol 111:104304. https://doi.org/10.1016/j.yexmp.2019.104304
Elrashidy RA, Kavran M, Asker ME, Mohamed HE, Daneshgari F, Liu G (2019) Smooth muscle-specific deletion of MnSOD exacerbates diabetes-induced bladder dysfunction in mice. Am J Physiol Renal Physiol 317:F906–F912. https://doi.org/10.1152/ajprenal.00221.2019
Evcim AS, Micili SC, Karaman M, Erbil G, Guneli E, Gidener S, Gumustekin M (2015) The role of Rac1 on carbachol-induced contractile activity in detrusor smooth muscle from streptozotocin-induced diabetic rats. Basic Clin Pharmacol Toxicol 116:476–484. https://doi.org/10.1111/bcpt.12346
Fukumoto Y, Yoshida M, Dokita S, Kamai T, Weiss RM, Latifpour J (1993) The reversal effect of insulin on diabetes-induced alterations in beta adrenergic and muscarinic receptors in rat prostate. J Urol 149:1602–1606. https://doi.org/10.1016/s0022-5347(17)36459-5
Fukumoto Y, Yoshida M, Weiss RM, Latifpour J (1994) Reversability of diabetes- and diuresis-induced alterations in rat bladder dome muscarinic receptors. Diabetes 43:819–826. https://doi.org/10.2337/diab.43.6.819
Goins WF, Yoshimura N, Phelan MW, Yokoyama T, Fraser MO, Ozawa H, Bennett N Jr, De Groat WC, Glorioso JC, Chancellor MB (2001) Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J Urol 165:1748–1754. https://doi.org/10.1016/S0022-5347(05)66407-5
Gotoh D, Torimoto K, Tatsumi Y, Hori S, Yamada A, Miyake M, Morizawa Y, Aoki K, Tanaka N, Hirayama A, Fujimoto K (2018) Tadalafil, a phosphodiesterase type 5 inhibitor, improves bladder blood supply and restores the initial phase of lower urinary tract dysfunction in diabetic rats. Neurourol Urodyn 37:666–672. https://doi.org/10.1002/nau.23372
Gotoh D, Torimoto K, Iwasaki H, Iwamoto T, Morizawa Y, Hori S, Miyake M, Tanaka N, Hirayama A, Nishi E, Fujimoto K (2019) Tadalafil, a phosphodiesterase type 5 inhibitor, restores urethra and detrusor function in the initial phase of diabetes in rats. Low Urin Tract Symptoms 11:241–247. https://doi.org/10.1111/luts.12272
Gotoh D, Cao N, Alexandre EC, Saito T, Morizawa Y, Hori S, Miyake M, Torimoto K, Fujimoto K, Yoshimura N (2021) Effects of low-dose insulin or a soluble guanylate cyclase activator on lower urinary tract dysfunction in streptozotocin-induced diabetic rats. Life Sci 286:120001. https://doi.org/10.1016/j.lfs.2021.120001
Gousse A, Yoshida M, Weiss RM, Latifpour J (1991) Beta adrenergic receptor alterations in diabetic rat prostate: effects of insulin and dietary myoinositol. Prostate 19:121–131. https://doi.org/10.1002/pros.2990190205
Gratzke C, Bachmann A, Descazeaud A, Drake MJ, Madersbacher S, Mamoulakis C, Oelke M, Tikkinen KA, Gravas S (2015) EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 67:1099–1109. https://doi.org/10.1016/j.eururo.2014.12.038
Gu B, Wu G, Si J, Xu Y, Andersson KE (2012) Improving voiding efficiency in the diabetic rat by a 5-HT1A serotonin receptor agonist. Neurourol Urodyn 31:168–173. https://doi.org/10.1002/nau.21182
Ha US, Bae WJ, Kim SJ, Yoon BI, Jang H, Hong SH, Lee JY, Hwang SY, Kim SW (2013) Protective effect of cyanidin-3-O-beta-D-glucopyranoside fraction from mulberry fruit pigment against oxidative damage in streptozotocin-induced diabetic rat bladder. Neurourol Urodyn 32:493–499. https://doi.org/10.1002/nau.22334
Han X, Gao Y, Yin X, Wang S, Zhang X, Chen Q (2021) Effect of electroacupuncture on bladder dysfunction via regulation of MLC and MLCK phosphorylation in a rat model of type 2 diabetes mellitus. Evid Based Complement Alternat Med 2021:5558890. https://doi.org/10.1155/2021/5558890
Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW (2019) Global trends in diabetes complications: a review of current evidence. Diabetologia 62:3–16. https://doi.org/10.1007/s00125-018-4711-2
He Y, Wu X, Cao Y, Hou Y, Chen H, Wu L, Lu L, Zhu W, Gu Y (2016) Daidzein exerts anti-tumor activity against bladder cancer cells via inhibition of FGFR3 pathway. Neoplasma 63:523–531
Höfner K, Burkart M, Jacob G, Jonas U (2010) Symptomatic and quality of life response to tolterodine in subgroups of men with overactive bladder symptoms and presumed non-obstructive benign prostatic hyperplasia. World J Urol 28:353–357. https://doi.org/10.1007/s00345-009-0460-7
Hotta Y, Takahashi S, Tokoro M, Naiki-Ito A, Maeda K, Kawata R, Kataoka T, Ohta Y, Hamakawa T, Takahashi S, Yasui T, Kimura K (2020) Anagliptin, a dipeptidyl peptidase-4 inhibitor, improved bladder function and hemodynamics in rats with bilateral internal iliac artery ligation. Neurourol Urodyn 39:1922–1929. https://doi.org/10.1002/nau.24449
Hsieh TH, Lin YT, Chen SC, Peng CW (2016) Chronic pudendal neuromodulation using an implantable microstimulator improves voiding function in diabetic rats. J Neural Eng 13:046001. https://doi.org/10.1088/1741-2560/13/4/046001
Huang Y, Gao J, Zhou Y, Wu S, Shao Y, Xue H, Shen B, Ding L, Wei Z (2020) Therapeutic effect of integrin-linked kinase gene-modified bone marrow-derived mesenchymal stem cells for streptozotocin-induced diabetic cystopathy in a rat model. Stem Cell Res Ther 11:278. https://doi.org/10.1186/s13287-020-01795-4
Hughes FM Jr, Hirshman NA, Inouye BM, Jin H, Stanton EW, Yun CE, Davis LG, Routh JC, Purves JT (2019) NLRP3 promotes diabetic bladder dysfunction and changes in symptom-specific bladder innervation. Diabetes 68:430–440. https://doi.org/10.2337/db18-0845
Ibrahim H, Maignel J, Hornby F, Daly D, Beard M (2022) BoNT/A in the urinary bladder—more to the story than silencing of cholinergic nerves. Toxins 14:53
Ikeda K, Wada Y, Foster HE Jr, Wang Z, Weiss RM, Latifpour J (2000) Experimental diabetes-induced regression of the rat prostate is associated with an increased expression of transforming growth factor-beta. J Urol 164:180–185
Irwin DE, Milsom I, Hunskaar S, Reillly K, Kopp Z, Herschorn S, Coyne K, Kelleher C, Hampel C, Artibani W, Abrams P (2006) Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 50:1306–1315. https://doi.org/10.1016/j.eururo.2006.09.019
Jia GL, Huang Q, Cao YN, Xie CS, Shen YJ, Chen JL, Lu JH, Zhang MB, Li J, Tao YX (2020) Cav-1 participates in the development of diabetic neuropathy pain through the TLR4 signaling pathway. J Cell Physiol 235:2060–2070
Jiang YJ, Gong DX, Liu HB, Yang CM, Sun ZX, Kong CZ (2008) Ability of α-lipoic acid to reverse the diabetic cystopathy in a rat model. Acta Pharmacol Sin 29:713–719. https://doi.org/10.1111/j.1745-7254.2008.00790.x
Kageyama S, Beppu M, Ohnogi H, Miyazaki S, Haruno A, Ito Y, Yamada S (2018) Clinical effects of formulated food of peucedanum japonicum extract and saw palmetto extract in male patients with lower urinary tract symptoms. Low Urin Tract Symptoms 10:167–174. https://doi.org/10.1111/luts.12155
Kamai T, Fukumoto Y, Gousse A, Yoshida M, Davenport TA, Weiss RM, Latifpour J (1994) Diabetes-induced alterations in the properties of muscarinic cholinergic receptors in rat vas deferens. J Urol 152:1017–1021. https://doi.org/10.1016/s0022-5347(17)32646-0
Kanika ND, Chang J, Tong Y, Tiplitsky S, Lin J, Yohannes E, Tar M, Chance M, Christ GJ, Melman A, Davies KD (2011) Oxidative stress status accompanying diabetic bladder cystopathy results in the activation of protein degradation pathways. BJU Int 107:1676–1684. https://doi.org/10.1111/j.1464-410X.2010.09655.x
Kong L-L, Wu H, Cui W-P, Zhou W-H, Luo P, Sun J, Yuan H, Miao L-N (2013) Advances in murine models of diabetic nephropathy. J Diabetes Res 2013:797548–797548. https://doi.org/10.1155/2013/797548
Koo HP, Santarosa RP, Buttyan R, Shabisgh R, Olsson CA, Kaplan SA (1993) Early molecular changes asociated with streptozotocin-induced diabetic bladder hypertrophy in the rat. Urol Res 21:375–381. https://doi.org/10.1007/BF00300072
Laddha AP, Kulkarni YA (2021b) Daidzein mitigates myocardial injury in streptozotocin-induced diabetes in rats. Life Sci 265:118779. https://doi.org/10.1016/j.lfs.2020.118779
Laddha AP, Kulkarni YA (2022) Daidzein attenuates urinary bladder dysfunction in streptozotocin-induced diabetes in rats by NOX-4 and RAC-1 inhibition. Naunyn-Schmiedeberg's Arch Pharmacol this issue. https://doi.org/10.1007/s00210-022-02246-y
Laddha AP, Kulkarni YA (2021) Daidzein ameliorates diabetic retinopathy in experimental animals. Life Sci 265:118779
Latifpour J, Gousse A, Yoshida M, Weiss RM (1991) Muscarinic receptors in diabetic rat prostate. Biochem Pharmacol 42(Suppl):S113-119. https://doi.org/10.1016/0006-2952(91)90400-y
Latifpour J, Gousse A, Yoshida M, Weiss RM (1992) Effect of insulin and dietary myoinositol on muscarinic receptor alterations in diabetic rat bladder. J Urol 147:760–763. https://doi.org/10.1016/s0022-5347(17)37374-3
Lee SJ, Karter AJ, Thai JN, Van Den Eeden SK, Huang ES (2013) Glycemic control and urinary incontinence in women with diabetes mellitus. J Womens Health 22:1049–1055. https://doi.org/10.1089/jwh.2012.4093
Lee YC, Hsieh TJ, Tang FH, Jhan JH, Lin KL, Juan YS, Wang HS, Long CY (2021) Therapeutic effect of low intensity extracorporeal shock wave therapy (Li-ESWT) on diabetic bladder dysfunction in a rat model. Int J Med Sci 18:1423–1431. https://doi.org/10.7150/ijms.55274
Leiria LO, Silva FH, Davel APC, Alexandre ED, Calixto MC, De Nucci G, Monica FZ, Antunes E (2014) The soluble guanylyl cyclase activator GAY 60–2770 ameliorates overactive bladder in obese mice. J Urol 191:539–547. https://doi.org/10.1016/j.juro.2013.09.020
Lenherr SM, Clemens JQ, Braffett BH, Dunn RL, Cleary PA, Kim C, Herman WH, Hotaling JM, Jacobson AM, Brown JS, Wessells H, Sarma AV (2016) Glycaemic control and risk of incident urinary incontinence in women with Type 1 diabetes: results from the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabet Med 33:1528–1535. https://doi.org/10.1111/dme.13126
Levay M, Krobert KA, Wittig K, Voigt N, Bermudez M, Wolber G, Dobrev D, Levy FO, Wieland T (2013) NSC23766, a widely used inhibitor of Rac1 activation, additionally acts as a competitive antagonist at muscarinic acetylcholine receptors. J Pharmacol Exp Ther 347:69–79. https://doi.org/10.1124/jpet.113.207266
Lewis RW, Sadovsky R, Eardley I, O’Leary M, Seftel A, Wang WC, Shen W, Walker DJ, Wong DG, Ahuja S (2005) The efficacy of tadalafil in clinical populations. J Sex Med 2:517–531. https://doi.org/10.1111/j.1743-6109.2005.00068.x
Li PC, Liu LF, Jou MJ, Wang HK (2016) The GLP-1 receptor agonists exendin-4 and liraglutide alleviate oxidative stress and cognitive and micturition deficits induced by middle cerebral artery occlusion in diabetic mice. BMC Neurosci 17:37. https://doi.org/10.1186/s12868-016-0272-9
Li B, Yu Q, Wang R, Gratzke C, Wang X, Spek A, Herlemann A, Tamalunas A, Strittmatter F, Waidelich R, Stief CG, Hennenberg M (2020) Inhibition of female and male human detrusor smooth muscle contraction by the Rac inhibitors EHT1864 and NSC23766. Front Pharmacol 11 https://doi.org/10.3389/fphar.2020.00409
Lin CF, Chueh TH, Chung CH, Chung SD, Chang TC, Chien CT (2020) Sulforaphane improves voiding function via the preserving mitochondrial function in diabetic rats. J Formos Med Assoc 119:1422–1430. https://doi.org/10.1016/j.jfma.2019.11.017
Lin K-L, Chueh K-S, Lu J-H, Chuang S-M, Wu B-N, Lee Y-C, Wu Y-H, Shen M-C, Sun T-W, Long C-Y (2021) Low intensity extracorporeal shock wave therapy as a novel treatment for stress urinary incontinence: a randomized-controlled clinical study. Medicina 57:947
Liu G, Daneshgari F (2014) Diabetic bladder dysfunction. Chin Med J 127:1357–1364
Liu YD, Zhang SC, Xue J, Wei ZQ, Shen BX, Ding LC (2019) Caffeine improves bladder function in streptozotocin-induced diabetic rats. Neurourol Urodyn 38:81–86. https://doi.org/10.1002/nau.23799
Livingstone DE, Barat P, Di Rollo EM, Rees GA, Weldin BA, Rog-Zielinska EA, MacFarlane DP, Walker BR, Andrew R (2015) 5α-Reductase type 1 deficiency or inhibition predisposes to insulin resistance, hepatic steatosis, and liver fibrosis in rodents. Diabetes 64:447–458. https://doi.org/10.2337/db14-0249
Longhurst PA, Kauer J, Levin RM (1991) The ability of insulin treatment to reverse or prevent the changes in urinary bladder function caused by streptozotocin-induced diabetes mellitus. Gen Pharmacol 22:305–311
Longhurst PA, Levendusky MC, Bezuijen MWF (2004) Diabetes mellitus increases the rate of development of decompensation in rats with outlet obstruction. J Urol 171:933–937
Lua LL, Pathak P, Dandolu V (2017) Comparing anticholinergic persistence and adherence profiles in overactive bladder patients based on gender, obesity, and major anticholinergic agents. Neurourol Urodyn 36:2123–2131. https://doi.org/10.1002/nau.23256
Masuda K, Aizawa N, Watanabe D, Okegawa T, Kume H, Igawa Y, Fukuhara H (2020) Pathophysiological changes of the lower urinary tract behind voiding dysfunction in streptozotocin-induced long-term diabetic rats. Sci Rep 10:4182. https://doi.org/10.1038/s41598-020-61106-y
Matsumoto T, Hatakeyama S, Imai A, Tanaka T, Hagiwara K, Konishi S, Okita K, Yamamoto H, Tobisawa Y, Yoneyama T, Yoneyama T, Hashimoto Y, Koie T, Nakaji S, Ohyama C (2019) Relationship between oxidative stress and lower urinary tract symptoms: results from a community health survey in Japan. BJU Int 123:877–884. https://doi.org/10.1111/bju.14535
Matsuo T, Miyata Y, Araki K, Mukae Y, Otsubo A, Ohba K, Sakai H (2020) Efficacy of tadalafil therapy and changes in oxidative stress levels in male patients with lower urinary tract symptoms and overactive bladder. Low Urin Tract Symptoms 12:47–53. https://doi.org/10.1111/luts.12283
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A (2022) 2022 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 24:4–131. https://doi.org/10.1093/eurheartj/ehab368
Melman A, Zotova E, Kim M, Arezzo J, Davies K, DiSanto M, Tar M (2009) Longitudinal studies of time-dependent changes in both bladder and erectile function after streptozotocin-induced diabetes in Fischer 344 male rats. BJU Int 104:1292–1300. https://doi.org/10.1111/j.1464-410X.2009.08573.x
Michel MC, Mehlburger L, Schumacher H, Bressel HU, Goepel M (2000) Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J Urol 163:1725–1729
Michel MC, Chess-Williams R, Hegde SS (2015) Are blood vessels a target to treat lower urinary tract dysfunction? Naunyn-Schmiedeberg’s Arch Pharmacol 388:687–694. https://doi.org/10.1007/s00210-015-1137-y
Michel MC, Mayoux E, Vallon V (2015) A comprehensive review of the pharmacodynamics of the SGLT2 inhibitor empagliflozin in animals and humans. Naunyn Schmiedebergs Arch Pharmacol 388:801–816. https://doi.org/10.1007/s00210-015-1134-1
Michel MC, Brunner HR, Foster C, Huo Y (2016) Angiotensin II type 1 receptor antagonists in animal models of vascular, cardiac, metabolic and renal disease. Pharmacol Ther 164:1–81. https://doi.org/10.1016/j.pharmthera.2016.03.019
Michel MC, Schumacher H, Mehlburger L, de la Rosette JJMCH (2020) Factors associated with nocturia-related quality of life in men with lower urinary tract symptoms and treated with tamsulosin oral controlled absorption system in a noniInterventional study. Front Pharmacol 11:816. https://doi.org/10.3389/fphar.2020.00816
Michel MC, Yesilyurt ZE, Öztürk G, Arioglu Inan E (2021) Empagliflozin and linagliptin do not affect urinary bladder enlargement in a rat model of type 1 diabetes. Br J Pharmacol 178:4977–4978. https://doi.org/10.1111/bph.15648
Mohammad G, Duraisamy AJ, Kowluru A, Kowluru RA (2019) Functional regulation of an oxidative stress mediator, Rac1, in diabetic retinopathy. Mol Neurobiol 56:8643–8655. https://doi.org/10.1007/s12035-019-01696-5
Mosli HH, Esmat A, Atawia RT, Shoieb SM, Mosli HA, Abdel-Naim AB (2015) Metformin attenuates testosterone-induced prostatic hyperplasia in rats: a pharmacological perspective. Sci Rep 5:15639. https://doi.org/10.1038/srep15639
Mossa AH, Galan A, Cammisotto PG, Velasquez Flores M, Shamout S, Barcelona P, Saragovi HU, Campeau L (2020) Antagonism of proNGF or its receptor p75NTR reverses remodelling and improves bladder function in a mouse model of diabetic voiding dysfunction. Diabetologia 63:1932–1946. https://doi.org/10.1007/s00125-020-05222-4
Navarro JF, Mora C (2005) Role of inflammation in diabetic complications. Nephrol Dial Transplant 20:2601–2604
Niclauß N, Michel-Reher MB, Alewijnse AE, Michel MC (2006) Comparison of three radioligands for the labelling of human ß-adrenoceptor subtypes. Naunyn-Schmiedeberg’s Arch Pharmacol 374:99–105. https://doi.org/10.1007/s00210-006-0104-z
Nirmal J, Tyagi P, Chuang YC, Lee WC, Yoshimura N, Huang CC, Rajaganapathy B, Chancellor MB (2014) Functional and molecular characterization of hyposensitive underactive bladder tissue and urine in streptozotocin-induced diabetic rat. PLoS ONE 9:e102644. https://doi.org/10.1371/journal.pone.0102644
Obata J, Matsumoto K, Yamanaka H, Ninomiya A, Nakamura S (2013) Who would benefit from solifenacin add-on therapy to tamsulosin for overactive bladder symptoms associated with nenign prostatic hyperplasia? Low Urin Tract Symptoms 5:145–149. https://doi.org/10.1111/luts.12005
Ochodnicky P, Cruz CD, Yoshimura N, Michel MC (2011) Nerve growth factor in bladder dysfunction: contributing factor, biomarker and therapeutic target. Neurourol Urodyn 30:1227–1241. https://doi.org/10.1002/nau.21022
Oelke M , Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, N'dow J, Nordling J, de la Rosette JJ (2013) EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 64:118–40. https://doi.org/10.1016/j.eururo.2013.03.004
Oger-Roussel S, Behr-Roussel D, Caisey S, Kergoat M, Charon C, Audet A, Bernabé J, Alexandre L, Giuliano F (2014) Bladder and erectile dysfunctions in the Type 2 diabetic Goto-Kakizaki rat. Am J Physiol Regul Integr Comp Physiol 306:R108-117. https://doi.org/10.1152/ajpregu.00033.2013
Oliveira AL, de Oliveira MG, Medeiros ML, Mónica FZ, Antunes E (2021) Metformin abrogates the voiding dysfunction induced by prolonged methylglyoxal intake. Eur J Pharmacol 910:174502. https://doi.org/10.1016/j.ejphar.2021.174502
O’Mara AE, Johnson JW, Linderman JD, Brychta RJ, McGehee S, Fletcher LA, Fink YA, Kapuria D, Cassimatis TM, Kelsey N, Cero C, Abdul-Sater Z, Piccinini F, Baskin AS, Leitner BP, Cai H, Millo CM, Dieckmann W, Walter M, Javitt NB, Rotman Y, Walter PJ, Ader M, Bergman RN, Herscovitch P, Chen KY, Cypess AM (2020) Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J Clin Investig 130:2209–2219. https://doi.org/10.1172/JCI131126
Öztürk B, Centinkaya M, Gür S, Ugurlu Ö, Öztekin V, Aki FT (2002) The early effects of partial outflow obstruction on contractile properties of diabetic and non-diabetic rat bladder. Urol Res 30:178–184. https://doi.org/10.1007/s00240-002-0247-4
Park M-H, Ju J-W (2013) Daidzein inhibits carbohydrate digestive enzymes in vitro and alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol 712:48–52
Park SA, Choi M-S, Cho S-Y, Seo J-S, Jung UJ, Kim M-J, Sung M-K, Park YB, Lee M-K (2006) Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci 79:1207–1213
Poladia DP, Bauer JA (2004) Oxidant driven signaling pathways during diabetes: role of Rac1 and modulation of protein kinase activity in mouse urinary bladder. Biochimie 86:543–551. https://doi.org/10.1016/j.biochi.2004.07.008
Rahman A, Davis B, Lövdahl C, Hanumaiah VT, Feil R, Brakebusch C, Arner A (2014) The small GTPase Rac1 is required for smooth muscle contraction. J Physiol 592:915–926. https://doi.org/10.1113/jphysiol.2013.262998
Rahnama’i MS, Ückert S, Hohnen R, van Koeveringe GA (2013) The role of phosphodiesterases in bladder pathophysiology. Nat Rev Urol 10:414–424. https://doi.org/10.1038/nrurol.2013.101
Ramage AG (2006) The role of central 5-hydroxytryptamine (5-HT, serotonin) receptors in the control of micturition. Br J Pharmacol 147:S120–S131
Robb EL, Stuart JA (2014) Multiple phytoestrogens inhibit cell growth and confer cytoprotection by inducing manganese superoxide dismutase expression. Phytother Res 28:120–131
Roghani M, Vaez Mahdavi MR, Jalali-Nadoushan MR, Baluchnejadmojarad T, Naderi G, Roghani-Dehkordi F, Taghi Joghataei M, Kord M (2013) Chronic administration of daidzein, a soybean isoflavone, improves endothelial dysfunction and attenuates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res 27:112–117
Saito M, Nishi K, Fukumoto Y, Weiss RM, Latifpour J (1996) Characterization of endothelin receptors in streptozotocin-induced diabetic rat vas deferens. Biochem Pharmacol 52:1593–1598. https://doi.org/10.1016/s0006-2952(96)00565-5
Saito M, Wada Y, Ikeda K, Wang Z, Foster HE Jr, Smith SD, Weiss RM, Latifpour J (2000) Expression of endothelin receptor subtypes and their messenger RNAs in diabetic rat prostate: effect of insulin treatment. Mol Cell Biochem 210:1–12. https://doi.org/10.1023/a:1007041909477
Saito M, Wada Y, Ikeda K, Wang Z, Smith SD, Foster HE, Nishi K, Weiss RM, Latifpour J (2000) Gene expression, localization, and pharmacological characterization of endothelin receptors in diabetic rat bladder dome. Eur J Pharmacol 387:253–263. https://doi.org/10.1016/s0014-2999(99)00753-0
Sasaki K, Chancellor MB, Phelan MW, Yokoyama T, Fraser MO, Seki S, Kubo K, Kumon H, De Groat WC, Yoshimura N (2002) Diabetic cystopathy correlates with a long-term decrease in nerve growth factor levels in the bladder and lumbosacral dorsal root ganglia. J Urol 168:1259–1264. https://doi.org/10.1016/S0022-5347(05)64636-8
Sasaki K, Chancellor MB, Goins WF, Phelan MW, Glorioso JC, De Groat WC, Yoshimura N (2004) Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes 53:2723–2730
Schneider T, Marschall-Kehrel D, Hanisch JU, Michel MC (2013) Does concomitant diabetes affect treatment responses in overactive bladder patients? Int J Clin Pract 67:1138–1143. https://doi.org/10.1111/ijcp.12196
Sekido N, Otsuki T, Kida J, Mashimo H, Wakamatsu D, Okada H, Matsuya H (2020) EP2 and EP3 receptors as therapeutic targets for underactive bladder/detrusor underactivity due to diabetic cystopathy in a type 1 diabetic rat model. Low Urin Tract Symptoms 12:285–291. https://doi.org/10.1111/luts.12317
Selig DJ, Brown AJ, DeLuca JP, Kress AT, Livezey JR, Oliver TG, Por ED, Thelus Jean R (2020) Risk of type 2 diabetes mellitus by antimuscarinic agents among adult females receiving care in the military health system. Pharmacoepidemiol Drug Saf 29:1605–1615. https://doi.org/10.1002/pds.5090
Shin JH, Ryu CM, Ju H, Yu HY, Song S, Hong KS, Chung HM, Park J, Shin DM, Choo MS (2020) Therapeutic efficacy of human embryonic stem cell-derived multipotent stem/stromal cells in diabetic detrusor underactivity: a preclinical study. J Clin Med 92853. https://doi.org/10.3390/jcm9092853
Steinbacher BC Jr, Nadelhaft I (1998) Increased levels of nerve growth factor in the urinary bladder and hypertrophy of dorsal root ganglion neurons in the diabetic rat. Brain Res 782:255–260. https://doi.org/10.1016/S0006-8993(97)01287-0
Su S-J, Yeh T-M, Lei H-Y, Chow N-H (2000) The potential of soybean foods as a chemoprevention approach for human urinary tract cancer. Clin Cancer Res 6:230–236
Szasz T, Wenceslau CF, Burgess B, Nunes KP, Webb RC (2016) Toll-like receptor 4 activation contributes to diabetic bladder dysfunction in a murine model of type 1 diabetes. Diabetes 65:3754–3764. https://doi.org/10.2337/db16-0480
Tatemichi S, Tsuchioka K, Yonekubo S, Maruyama K, Kobayashi M (2015) Effects of Silodosin, an α1A-adrenoceptor antagonist, and distigmine, an acetylcholinesterase inhibitor, and their combined effects on impaired voiding function in Zucker Diabetic Fatty rats. Pharmacology 95:285–292. https://doi.org/10.1159/000398811
Thurmond P, Yang JH, Azadzoi KM (2016) LUTS in pelvic ischemia: a new concept in voiding dysfunction. Am J Physiol 310:F738–F743. https://doi.org/10.1152/ajprenal.00333.2015
Tong YC, Cheng JT (2005) Changes in bladder nerve-growth factor and p75 NTR genetic expression in streptozotocin-induced diabetic rats. BJU Int 96:1392–1396. https://doi.org/10.1111/j.1464-410X.2005.05854.x
Torimoto K, Hirao Y, Matsuyoshi H, de Groat WC, Chancellor MB, Yoshimura N (2005) alpha1-Adrenergic mechanism in diabetic urethral dysfunction in rats. J Urol 173:1027–1032. https://doi.org/10.1097/01.ju.0000146268.45662.36
Tsounapi P, Honda M, Hikita K, Sofikitis N, Takenaka A (2019) Oxidative stress alterations in the bladder of a short-period type 2 diabetes rat model: antioxidant treatment can be beneficial for the bladder. In Vivo 33:1819–1826. https://doi.org/10.21873/invivo.11674
Tu H, Cao N, Gu B, Si J, Chen Z, Andersson KE (2015) Serotonin (5-HT)2A/2C receptor agonist (2,5-dimethoxy-4-idophenyl)-2-aminopropane hydrochloride (DOI) improves voiding efficiency in the diabetic rat. BJU Int 116:147–155. https://doi.org/10.1111/bju.12684
Valeri A, Fiorenzani P, Rossi R, Aloisi AM, Valoti M, Pessina F (2012) The soy phytoestrogens genistein and daidzein as neuroprotective agents against anoxia-glucopenia and reperfusion damage in rat urinary bladder. Pharmacol Res 66:309–316
Van Den Eeden SK, Sarma AV, Rutledge BN, Cleary PA, Kusek JW, Nyberg LM, McVary KT, Wessells H (2009) Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care 32:664–670. https://doi.org/10.2337/dc07-2375
Wang Z, Ikeda K, Wada Y, Foster HE, Weiss RM, Latifpour J (2000) Expression and localization of basic fibroblast growth factor in diabetic rat prostate. BJU Int 85:945–952. https://doi.org/10.1046/j.1464-410x.2000.00597.x
Wang XM, Zhang MX, Zhao L, Han B, Xu P, Xue M (2010) The Starling mechanism of the urinary bladder contractile function and the influence of hyperglycemia on diabetic rats. J Diabetes Complicat 24:121–128. https://doi.org/10.1016/j.jdiacomp.2009.06.002
Wang Z, Cheng Z, Cristofaro V, Li J, Xiao X, Gomez P, Ge R, Gong E, Strle K, Sullivan MP, Adam RM, White MF, Olumi AF (2012) Inhibition of TNF-α improves the bladder dysfunction that is associated with type 2 diabetes. Diabetes 61:2134–2145. https://doi.org/10.2337/db11-1763
Wang Y, Kunit T, Ciotkowska A, Rutz B, Schreiber A, Strittmatter F, Waidelich R, Liu C, Stief CG, Gratzke C, Hennenberg M (2015) Inhibition of prostate smooth muscle contraction and prostate stromal cell growth by the inhibitors of Rac, NSC23766 and EHT1864. Br J Pharmacol 172:2905–2917. https://doi.org/10.1111/bph.13099
Wang Y, Deng GG, Davies KP (2016) Novel insights into development of diabetic bladder disorder provided by metabolomic analysis of the rat nondiabetic and diabetic detrusor and urothelial layer. Am J Physiol-Endocrinol Metab 311:E471–E479. https://doi.org/10.1152/ajpendo.00134.2016
Wang HS, Oh BS, Wang B, Ruan Y, Zhou J, Banie L, Lee YC, Tamaddon A, Zhou T, Wang G, Lin G, Lue TF (2018) Low-intensity extracorporeal shockwave therapy ameliorates diabetic underactive bladder in streptozotocin-induced diabetic rats. BJU Int 122:490–500. https://doi.org/10.1111/bju.14216
Wang R, Yu Q, Wang X, Li B, Ciotkowska A, Rutz B, Wang Y, Stief CG, Hennenberg M (2020) Rac1 silencing, NSC23766 and EHT1864 reduce growth and actin organization of bladder smooth muscle cells. Life Sci 261:118468. https://doi.org/10.1016/j.lfs.2020.118468
Wang J, Dai L, Yue X, Shen C, Li T, Long L, Zhi Y, Wang Y, Shen G, Shi C, Liu Y, Fang Q, Li W (2021) IR-61 improves voiding function via mitochondrial protection in diabetic tats. Front Pharmacol 12:608637. https://doi.org/10.3389/fphar.2021.608637
Wang CC, Jiang YH, Kuo HC (2020a) The pharmacological mechanism of diabetes mellitus-associated overactive bladder and Its treatment with botulinum toxin A. Toxins (Basel) 12(3):186. https://doi.org/10.3390/toxins12030186
Wanner C, Heerspink HJL, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, Hantel S, Woerle H-J, Broedl UC, von Eynatten M, Groop P-H (2018) Empagliflozin and kidney function decline in patients with type 2 diabetes: a Slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol 29:2755–2769. https://doi.org/10.1681/asn.2018010103
Wei L, Lai EC, Kao-Yang YH, Walker BR, MacDonald TM, Andrew R (2019) Incidence of type 2 diabetes mellitus in men receiving steroid 5α-reductase inhibitors: population based cohort study. BMJ 365:l1204. https://doi.org/10.1136/bmj.l1204
WenBo W, Fei Z, YiHeng D, Wei W, TingMang Y, WenHao Z, QianRu L, HaiTao L (2017) Human umbilical cord mesenchymal stem cells overexpressing nerve growth factor ameliorate diabetic cystopathy in rats. Neurochem Res 42:3537–3547. https://doi.org/10.1007/s11064-017-2401-y
Whitmire W, Al-Gayyar MMH, Abdelsaid M, Yousufzai BK, El-Remessy AB (2011) Alteration of growth factors and neuronal death in diabetic retinopathy: what we have learned so far. Mol vis 17:300–308
Witte LPW, Chapple CR, de la Rosette JJMCH, Michel MC (2008) Cholinergic innervation and muscarinic receptors in the human prostate. Eur Urol 54:326–334. https://doi.org/10.1016/j.eururo.2007.12.007
World Health Organization (2016) Diabetes Fact Sheet.
Xiao N, Wang Z, Huang Y, Daneshgari F, Liu G (2013) Roles of polyuria and hyperglycemia in bladder dysfunction in diabetes. J Urol 189:1130–1136. https://doi.org/10.1016/j.juro.2012.08.222
Xiao N, Huang Y, Kavran M, Elrashidy RA, Liu G (2015) Short-term diabetes- and diuresis-induced alterations of the bladder are mostly reversible in rats. Int J Urol 22:410–415. https://doi.org/10.1111/iju.12695
Xu S-Z, Zhong W, Ghavideldarestani M, Saurabh R, Lindow SW, Atkin SL (2009) Multiple mechanisms of soy isoflavones against oxidative stress-induced endothelium injury. Free Radical Biol Med 47:167–175
Xue J, Liu Y, Zhang S, Ding L, Shen B, Shao Y, Wei Z (2021) Caffeine improves bladder function in diabetic rats via a neuroprotective effect. Exp Ther Med 21:501. https://doi.org/10.3892/etm.2021.9932
Yamada S, Takeuchi C, Oyunzul L, Ito Y (2009) Bladder angiotensin-II receptors: characterization and alteration in bladder outlet obstruction. Eur Urol 55:482–490. https://doi.org/10.1016/j.eururo.2008.03.015
Yesilyurt ZE, Erdogan BR, Karaomerlioglu I, Müderrisoglu AE, Michel MC, Arioglu Inan E (2019) Urinary bladder weight and function in a rat model of mild hyperglycemia and its treatment with dapagliflozin. Front Pharmacol 10:911. https://doi.org/10.3389/fphar.2019.00911
Yesilyurt ZE, Ertürk BM, Erdogan BR, Arioglu Inan E, Michel MC (2021) Effects of the sodium-glucose transporter 2 inhibitor empagliflozin on bladder sie, contraction and ralexation in a rat model of type 1 diabetes. Neurourol Urodyn 42(Suppl 2):S141–S142. https://doi.org/10.1002/nau.24746
Yi C-r, Wei Z-q, Deng X-l, Sun Z-y, Li X-r, Tian C-g (2006) Effects of coffee and caffeine on bladder dysfunction in streptozotocin-induced diabetic rats. Acta Pharmacol Sin 27:1037–1043
Yonekubo S, Tatemichi S, Maruyama K, Kobayashi M (2017) Alpha1A-adrenoceptor antagonist improves underactive bladder associated with diabetic cystopathy via bladder blood flow in rats. BMC Urol 17:64. https://doi.org/10.1186/s12894-017-0256-9
Yono M, Pouresmail M, Takahashi W, Flanagan JF, Weiss RM, Latifpour J (2005) Effect of insulin treatment on tissue size of the genitourinary tract in BB rats with spontaneously developed and streptozotocin-induced diabetes. Naunyn-Schmiedeberg’s Arch Pharmacol 372:251–255. https://doi.org/10.1007/s00210-005-0010-9
Yono M, Mane SM, Lin A, Weiss RM, Latifpour J (2008) Differential effects of diabetes induced by streptozotocin and that develops spontaneously on prostate growth in Bio Breeding (BB) rats. Life Sci 83:192–197. https://doi.org/10.1016/j.lfs.2008.06.006
Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z (2019) Stem cells: past, present, and future. Stem Cell Res Ther 10:1–22
Zhang H, Qiu X, Shindel AW, Ning H, Ferretti L, Jin X, Lin G, Lin CS, Lue TF (2012) Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type II diabetic rat model. Stem Cells Dev 21:1391–1400. https://doi.org/10.1089/scd.2011.0244
Funding
Open Access funding enabled and organized by Projekt DEAL. Work related to LUT dysfunction in diabetes in the authors’ labs is funded by TÜBITAK-SBAG 118S443 and 119S769 (to EAI) and Deutsche Forschungsgemeinschaft Mi 294/10–1 (to MCM). GL was supported by the National Institutes of Health (NIH) NIDDK grant R01-DK110567. No paper mills were used in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
EAI and MCM conceptualized the idea. BRE, GL and MCM performed the literature search and drafted section of the manuscript. All authors have read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
BRE, EAI, and GL do not report a conflict of interest. In the field of LUT dysfunction, MCM has been a consultant and/or speaker for Apogepha, Astellas, Dr. Willmar Schwabe, GSK and Sanofi-Aventis.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erdogan, B.R., Liu, G., Arioglu-Inan, E. et al. Established and emerging treatments for diabetes-associated lower urinary tract dysfunction. Naunyn-Schmiedeberg's Arch Pharmacol 395, 887–906 (2022). https://doi.org/10.1007/s00210-022-02249-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02249-9