Abstract
Despite the fact that environmental pollution has been implicated in the global rise of diabetes, the research on the impact of emerging pollutants such as novel flame retardants remains limited. In line with the shift towards the use of non-animal approaches in toxicological testing, this study aimed to investigate the effects of two novel flame retardants tris(1,3-dichloro-2-propyl) phosphate (TDCIPP) and triphenyl phosphate (TPhP) in rat (INS1E) and human (NES2Y) pancreatic beta-cell lines. One-week exposure to 1 μM and 10 μM TDCIPP and TPhP altered intracellular insulin and proinsulin levels, but not the levels of secreted insulin (despite the presence of a statistically insignificant trend). The exposures also altered the protein expression of several factors involved in beta-cell metabolic pathways and signaling, including ATP citrate lyase, isocitrate dehydrogenase 1, perilipins, glucose transporters, ER stress-related factors, and antioxidant enzymes. This study has brought new and valuable insights into the toxicity of TDCIPP and TPhP on beta-cell function and revealed alterations that might impact insulin secretion after more extended exposure. It also adds to the scarce studies using in vitro pancreatic beta-cells models in toxicological testing, thereby promoting the development of non-animal testing strategy for identifying pro-diabetic effects of chemical pollutants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental pollution has become an admitted factor in the development of worldwide diabetes epidemics (Hoyeck et al. 2022). For instance, multiple reviews mostly based on epidemiological or in vivo animal studies describe persistent organic pollutants (Kim et al. 2019; Lee et al. 2018), air pollution (Yang et al. 2020), or pollutant-affected gut microbiota (Bailey et al. 2020) to play a role in the development of type-2 diabetes mellitus. Data also exist connecting persistent organic pollutants and type-1 diabetes (Bresson et al. 2022). A recent review highlights the associations between exposure to several environmental pollutants, such as phthalates or polybrominated diphenyl ethers (PBDEs), and the development of gestational diabetes mellitus (Yao et al. 2023). Among the possible mechanisms involved, increased oxidative stress in pancreatic beta-cells (Hoyeck et al. 2022; Jiang et al. 2021; Loiola et al. 2016; Marroqui et al. 2018; Park et al. 2020) or decreased insulin production (Hoyeck et al. 2022; Pavlikova et al. 2019, 2023) emerge from the literature in connection with type 2 diabetes development. However, studies remain scarce for many emerging pollutants, such as novel flame retardants (FRs).
FR chemicals are added to consumer products to delay flammability but may pose public health concerns. For instance, the long-used brominated FRs, such as PBDEs, have been added to Annex A of the Stockholm Convention on persistent organic pollutants, meaning they should be eliminated from the market. To meet the flammability standards, novel FRs, such as organophosphorus FRs (OPFRs), are being used as replacements. However, an increasing number of studies point to possible health effects (such as endocrine disruption, male reproductive disorders, hepatotoxicity, or neurotoxicity) associated with exposure to several OPFRs (Bajard et al. 2019, 2021; Blum et al. 2019b; Dishaw et al. 2014; Zhao et al. 2022). In particular, a few reports indicate the possible effects of novel FRs with type 2 diabetes mellitus. For instance, exposure to triphenyl phosphate (TPhP) reduces insulin-induced glucose uptake by liver cell lines and induces insulin resistance in mice (Yue et al. 2023). TPhP exposure in mice fetus also impacts glucose clearance (Wang et al. 2019). Another two studies in mice show that exposure to a mixture of tris(1,3-dichloro-2-propyl) phosphate (TDCIPP), TPhP, and tricresyl phosphate may alter plasma insulin levels and glucose clearance in a sex- and diet-dependent way (Krumm et al. 2018; Walley et al. 2021). These limited studies alert on the possible impact of OPFRs on glucose homeostasis and the development of type 2 diabetes mellitus. However, more studies are required to confirm these effects and elucidate the underlying toxicological mechanisms.
For more than a decade, alternative approaches to animal testing, such as in vitro cell models, have been strongly encouraged and increasingly employed in toxicological studies due to ethical, efficacy, and financial issues (Krewski et al. 2010; Langley et al. 2007). In particular, in vitro cultures are largely used to study the ability of chemicals to induce hepatotoxicity (Gómez-Lechón MJ et al. 2014; Negi et al. 2021) or skin toxicity (Hoffmann et al. 2018; Kleinstreuer et al. 2018). Utilizing beta-cell lines is crucial to address sustainability and ethical concerns associated with using primary human islets from donors. However, in vitro systems for the pancreas remain remarkably limited. Currently, no in vitro model for Langerhans islets exists, and the islets used in ex vivo experiments are directly isolated from rodents or humans. In vitro systems used for diabetes research consist of a few pancreatic beta-cell lines available.
This study aimed to test the effects of TDCIPP and TPhP on a rat beta-cell line (INS1E) and a human pancreatic beta-cell line (NES2Y). After a one-week exposure, we examined the expression of proteins involved in insulin production, glucose transport, metabolism, and oxidative stress defense.
Materials and methods
Materials
TDCIPP (CAS# 13,674-87-8) was purchased from Tokyo Chemical Industry (TCI) (> 95% purity, Cat# P0269, Lot# EFBGE-LQ), and TPhP (CAS# 115-86-6) from Sigma-Aldrich (> 99% purity, Cat# 24–12-88, Lot# BCBW9556) and RPMI medium from Sigma-Aldrich (https://www.sigmaaldrich.com). We purchased primary antibodies for aconitase 2 (ab129069), ATP citrate lyase (ab40793), isocitrate dehydrogenase 1 (ab172964), glutathione reductase (ab16801), superoxide dismutase 1 (ab51254), binding immunoglobulin protein (ab21685), phosphorylated iron-responsive element 1 (S724) (ab48187), and hypoxanthine–guanine phosphoribosyltransferase (ab109021) from Abcam (www.abcam.com); primary antibodies for biliverdin reductase B (17,729-1-AP), glucose transporter 1 (121,829-1-AP), glucose transporter 2 (20,436-1-AP), perilipin 2 (15,294-1-AP), and perilipin 5 (26,951-1AP) from Fisher Scientific (https://www.thermofisher.cz); primary antibody for cyclic AMP-dependent transcription factor (HPA001562), and actin (a3853) from Sigma-Aldrich (https://www.sigmaaldrich.com); and primary antibody for phosphorylated protein kinase A (Thr197) from Cell Signaling (https://www.cellsignal.com). We used Pierce™ BCA Protein Assay Kit (https://www.thermofisher.cz) to determine protein concentrations.
Cell culture
Our experiments employed the rat pancreatic beta-cell line INS1E with glucose-stimulated insulin secretion (kindly provided by Dr. Claes B. Wollheim at the Centre Medical Universitaire de Genève, Geneva, Switzerland) and the human pancreatic beta-cell line NES2Y with constitutive insulin secretion (kindly provided by Dr. Roger F. James, Department of Infection, Immunity and Inflammation, University of Leicester). We used a medium based on RPMI 1640, containing phenol red, L-glutamine, sodium pyruvate, HEPES, penicillin, and streptomycin, and supplemented with 10% fetal bovine serum (FBS), as previously described (Pavlikova et al. 2015) for culturing the cells. We maintained cells in a humidified atmosphere of 5% CO2 in air at 37 °C.
Viability assessment (neutral red assay)
We performed a Neutral Red Assay to determine the viability of pancreatic beta-cells exposed for 48 h to the following concentrations of TDCIPP or TPhP: 10 nM, 100 nM, 1 μM, 10 μM, and 100 μM, as described before (Pavlikova et al. 2023).
One-week exposure to pollutants
We seeded beta-cells into three six-well plates using a density of 50 000 cells/well/3 ml of media for NES2Y cells and 150 000 cells/well/3 ml for INS1E cells. The media already contained the selected concentrations (1 μM or 10 μM) of pollutants (TDCIPP or TPhP) or DMSO as solvent control (the concentration of DMSO in the medium was 0.5%). After 4 days, we replaced the medium with a new one containing pollutants. After three more days (7 days total), we removed the medium, washed cells three times with PBS, added RIPA lysis buffer containing protease and phosphatase inhibitors, and stored the plates at −80 °C. The next day, we thawed the contents of the wells and moved them to Eppendorf tubes. After centrifuging the lysates, we transferred the supernatants into new Eppendorf tubes and stored them at −80 °C. The protein concentrations were quantified employing the BCA commercial kit.
Western blot
We performed western blots as described before (Pavlikova et al. 2023) with minor modifications. We separated 18 ug of proteins on 10% polyacrylamide gels and then blotted them onto a nitrocellulose membrane. We incubated membranes with the primary antibodies overnight at 4 °C on a shaker. We used primary antibodies at concentrations 1:1000 for all primary antibodies except for HPRT and BiP, where we used concentration 1:10,000. Primary antibodies against phosphorylated forms of proteins were diluted in 5% BSA; the rest of the primary antibodies were diluted in 5% low-fat milk.
ELISA: intracellular insulin
We employed commercial kits to determine intracellular insulin and proinsulin levels (https://www.mercodia.com/; 10-1232-01, 10-1250-01). We diluted our samples into 1 μg/μl stock solutions. We used those stock solutions for further dilution of samples to find the concentrations fitting into the calibration curve. After performing the ELISA experiment, we determined the protein concentration of the 1 μg/μl sample solutions and normalized the results from ELISA to those values.
ELISA: insulin secretion
We seeded INS1E cells into a 24-well plate at a density of 50 000 cells/well/750 μL. The media already contained the selected concentrations (1 μM or 10 μM) of pollutants (TDCIPP or TPhP) or DMSO as solvent control. After 7 days of exposure, we determined insulin secretion. We removed the medium, washed the cells with PBS, and added RPMI medium without glucose for 2 h. After 2 h, we removed the RPMI medium and added Krebs buffer containing glucose 2.5 mM, glucose 25 mM, or KCl 30 mM combined with glucose 2.5 mM. After 30 min, we collected the Krebs buffer, centrifuged it (5000 g/5 min), and determined insulin content using a commercial kit (https://www.mercodia.com/; 10-1250-01).
Use of large language models
During the preparation of this work, the authors used GPT Poe to improve the manuscript’s language. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Results
Cell viability
From the tested concentrations (10 nM, 100 nM, 1 μM, 10 μM, and 100 μM), only 100 μM of both flame retardants affected beta-cell viability measured using the Neutral Red Assay. After 48-h exposure, 100 μM TDCIPP significantly decreased INS1E cell viability (Fig. 1B) to 15% of control and NES2Y cell viability (Fig. 1C) to 38% of control; 100 μM TPhP significantly decreased INS1E cell viability (Fig. 1B) to 2% of control and NES2Y cell viability (Fig. 1C) to 22% of control. For the follow-up examination of effects on cell functions in one-week exposure, we chose the two highest non-cytotoxic concentrations from our scale: 1 μM and 10 μM.
shows the chemical structures of TDCIPP and TPhP (A), the viability of (B) rat pancreatic beta-cells INS1E and (C) human pancreatic beta-cells NES2Y when exposed to DMOSO (con), 10 nM, 100 nM, 1 μM, 10 μM, and 100 μM of TDCIPP and TPhP for 48 h detected by a Neutral Red Assay. The graph shows the average of three independent experiments ± SEM. *** means statistical significance (p <0.001) determined by ONE-WAY ANOVA (Dunnet’s test)
Proinsulin and intracellular insulin levels and insulin secretion
In INS1E cells, both TDCIPP and TPhP (Fig. 1A) significantly affected intracellular levels of proinsulin and insulin but had opposing effects (Fig. 2A). One-week exposure to 10 μM TDCIPP decreased proinsulin level to 80% of control and intracellular insulin level to 63% of control. On the other hand, 1-week exposure to 10 μM TPhP increased proinsulin level to 121% of control and intracellular insulin level to 142% of control.
A Shows the levels of intracellular insulin and proinsulin in rat pancreatic beta-cells INS1E exposed to 1 μM and 10 μM TDCIPP and TPhP for one week detected by ELISA. B Shows insulin secretion by rat pancreatic beta-cells INS1E exposed to 1 μM and 10 μM concentrations of TDCIPP and TPhP for 1 week detected by ELISA. The graph shows the mean of four independent experiments (2A) and two independent experiments (2B) ± SEM. ** means statistical significance (p < 0.01), * means statistical significance (p < 0.05) determined by ONE-WAY ANOVA (Dunnet’s test)
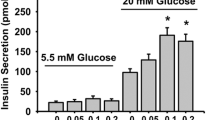
We then measured secreted insulin in INS1E cells. We have tested insulin secretion of the exposed cells stimulated by 2.5 mM glucose (hypoglycemia), 25 mM glucose (hyperglycemia), and 2.5 mM glucose in combination with 30 mM KCl, which stimulates insulin secretion independently on glucose intake. The exposure to pollutants did not significantly affect the levels of secreted insulin (Fig. 2B). Consistent with the effects on intracellular insulin, the exposure to 10 μM TPhP increased secreted insulin level when stimulated by high glucose concentration or KCl, but the changes were insignificant (Fig. 2B).
Effects on enzymes related to NADPH production
NADPH stimulates insulin secretion independently of the main glucose-stimulated signaling pathway (glucose uptake leads to increased ATP level and potassium channel closure resulting in voltage-gated calcium channel opening) by interaction with the insulin vesicle secretory mechanism, thereby amplifying the secretion process (Campbell and Newgard 2021; Zhang et al. 2021). The precise mechanism behind this phenomenon is unknown, but it was confirmed also in human beta-cells obtained from non-diabetic and type 2 diabetic donors (Ferdaoussi et al. 2015). Isocitrate dehydrogenase 1 (IDH1) generates NADPH from isocitrate in the cytosol, while aconitase-2 acts prior to IDH in the tricarboxylic acid cycle, facilitating the conversion of citrate to isocitrate.
The effects of flame retardants on IDH1 expression differed between rat and human beta-cells. In rat beta-cells (Fig. 3B), 10 μM TDCIPP significantly increased IDH1 protein expression to 211% of the control; 10 μM TPhP exposure showed no effect. In human beta-cells (Fig. 3D), both 10 μM TDCIPP and 10 μM TPhP significantly decreased IDH-1 protein expression to 67% and 71% of the control, respectively.
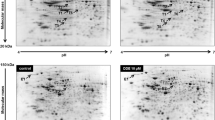
Shows the protein expression of (A, C) aconitase-2 (ACO-2) and (B, D) isocitrate dehydrogenase 1 (IDH1) in the rat (INS1E) pancreatic beta-cells and human (NES2Y) pancreatic beta-cells exposed to 1 μM and 10 μM TDCIPP and TPhP for 1 week. Actin was used as a loading control. The graphs represent an average of densitometric analyses of at least four western blots made of four independent sets of samples ± SEM. Below each graph, a representative western blot is shown. * means statistical significance (p < 0.05), ** means statistical significance (p< 0.01), *** means statistical significance (p < 0.001) determined by ONE-WAY ANOVA (Dunnet’s test)
.
Neither TPhP nor TDCIPP significantly altered the protein expression of aconitase-2 (ACO-2) in beta-cells (Fig. 3A, C), but there was a decreasing trend at 10 μM concentrations in human beta-cells (Fig. 3C).
Proteins involved in ER stress and translation
ER stress in pancreatic beta-cells leads to decreased insulin production (Fonseca et al. 2010; Wang et al. 2016). In response to ER stress, the cells initiate unfolded protein response (UPR), the chain of reactions that helps ER manage the accumulated unfolded proteins (Gong et al. 2017). The UPR comprises several branches; we tested the BiP–PERK–p-eIF2α branch and p-IRE1α branch (Cnop et al. 2017).
One-week exposure to flame retardants failed to alter the protein expression of BiP in both rat (Fig. 4A) and human beta-cells (Fig. 4D). The exposure to both chemicals (their 10 μM concentrations) altered the levels of the phosphorylated form of eIF2α (p-eIF2α), but the changes were inconsistent and not statistically significant in any cell line (Fig. 4C and 4F). In rat beta-cells, 10 μM TDCIPP significantly increased the phosphorylated form of IRE1α to 152% of control (Fig. 4B); in human beta-cells, p-IRE1α remained unchanged (Fig. 4E).
Shows the protein expression of (A, D) binding immunoglobulin protein (BiP/GRP-78), (B, E) inositol-requiring enzyme 1α (p-IRE1α), and (C, F) phosphorylated eukaryotic initiation factor 2 (p-eIF2α) in the rat (INS1E) pancreatic beta-cells and human (NES2Y) pancreatic beta-cells exposed to 1 μM and 10 μM TDCIPP and TPhP for 1 week. Actin or HPRT was used as a loading control. The graphs represent an average of densitometric analyses of at least four western blots made of three independent sets of samples ± SEM. Below each graph, a representative western blot is shown. ** means statistical significance (p < 0.01) determined by ONE-WAY ANOVA (Dunnet’s test)
Glucose transporters
Glucose transporters (GLUT) facilitate the transport of extracellular glucose into the cells. They represent the first step of glucose metabolism, which eventually leads to insulin secretion.
In rat beta-cells, exposure to pollutants failed to alter the protein expression of GLUT1 or GLUT2 (Fig. 5A and 5B). In human beta-cells, 10 μM TDCIPP increased GLUT1 protein expression to 249% of control (Fig. 5C) and decreased GLUT2 protein expression to 47% of control (Fig. 5D); both changes were statistically significant. In human beta-cells, 10 μM TPhP increased GLUT1 protein expression to 185% of control (Fig. 5C) and decreased GLUT2 protein expression to 67% of control; both changes were statistically significant. A consistent but insignificant trend was observed for both chemicals at 1 µM.
The protein expression of (A, C) glucose transporter 1 (GLUT1) and (B, D) glucose transporter 2 (GLUT2) in the rat (INS1E) and human (NES2Y) pancreatic beta-cells exposed to 1 μM and 10 μM TDCIPP and TPhP for 1 week. Actin or HPRT was used as a loading control. The graphs represent the mean ± SEM of densitometric analyses of at least four western blots from four independent sets of samples. Below each graph, a representative western blot is shown ± SEM. *means statistical significance (p < 0.05), **means statistical significance (p < 0.01), *** means statistical significance (p < 0.001) determined by ONE-WAY ANOVA (Dunnet's test)
Proteins involved in lipid synthesis and lipid droplet formation
ATP citrate lyase (ATP-CL) plays a critical role in cellular lipid production. It acts as a bridge between glucose and fatty acid metabolism by converting citrate into acetyl-CoA in the cytosol. Acetyl-CoA is a substrate for forming malonyl-CoA, which can stimulate insulin secretion (Aghelan et al. 2020).
One-week exposure to 10 μM TDCIPP significantly increased protein expression of ATP citrate lyase (ATP-CL) to 140% of control in rat (Fig. 6A) but not human beta-cells (Fig. 6D).
The protein expression of (A, D) ATP citrate lyase (ATP-CL), (B, E) perilipin 5 (PLIN5) and (C, F) perilipin 2 (PLIN2) in the rat (INS1E) pancreatic beta-cells and human (NES2Y) pancreatic beta-cells exposed to 1 μM and 10 μM concentrations of TDCIPP and TPhP for 1 week. Actin or HPRT was used as a loading control. The graphs represent an average of densitometric analyses of at least four western blots made of four independent sets of samples ± SEM. Below each graph, a representative western blot is shown. *means statistical significance (p < 0.05), ** means statistical significance (p < 0.01), ***means statistical significance (p < 0.001) determined by ONE-WAYANOVA (Dunnet's test)
Perilipins play an essential role in lipid droplet formation in a reaction to nutrition overload. In adipocytes, pollutants like DDE (Mullerova et al. 2016), methyl-Hg (Tinant et al. 2021), or PCB-138 (Kim et al. 2018) altered the expression of perilipins and acted as obesogens. Our study focused on two perilipins present in pancreatic beta-cells: perilipin 2 and perilipin 5.
10 μM TPhP decreased protein expression of perilipin 5 (PLIN5) in both beta-cell lines to 57% of control in rat beta-cells (Fig. 6B) and 77% of control in human beta-cells (Fig. 6E). Interestingly, in human (but not rat, Fig. 6C) beta-cells, the TPhP-induced PLIN5 downregulation is accompanied by a significant perilipin 2 (PLIN2) upregulation to 232% of control (Fig. 6F).
Proteins related to cAMP signaling
Pancreatic beta-cells rely on cAMP signaling to enhance insulin secretion. Within these cells, cAMP molecules serve as activators of protein kinase A (PKA) and exchange protein directly activated by cAMP (Epac), both of which play essential roles in regulating the exocytosis mechanism involved in insulin release (Pratt et al. 2019). We tested the protein expression of active (autophosphorylated at Thr197) protein kinase A (p-PKA). None of the tested concentrations altered the p-PKA levels in beta-cells (Fig. 7A and 7C).
Shows the protein expression of (A, C) phosphorylated form of protein kinase A (p-PKA) and (B, D) activating transcription factor 3 (ATF3) in the rat (INS1E) pancreatic beta-cells and human (NES2Y) pancreatic beta-cells exposed to 1 μM and 10 μM concentrations of TDCIPP and TPhP for 1 week. Actin or HPRT was used as a loading control. The graphs represent means ± SEM from at least three independent sets of samples. Below each graph, a representative western blot is shown. **means statistical significance (p < 0.01) determined by ONE-WAYANOVA (Dunnet’s test)
Activating transcription factor 3 (ATF3) is a cAMP-dependent transcription factor induced by various types of cellular stress, e.g., oxidative stress, ER stress, high glucose, or high fatty acids (Ku and Cheng 2020; Zmuda et al. 2010).
In human beta-cells, both 10 μM TDCIPP and 10 μM TPhP significantly decreased ATF3 protein expression: 10 μM TDCIPP to 43% of control and 10 μM TPhP to 47% of control (Fig. 7D).
Enzymes involved in oxidative stress defense
Pancreatic beta-cells are known to be highly susceptible to oxidative stress due to their relatively lower levels of antioxidant enzymes compared to other cell types (Lenzen 2017). In our study, we measured the levels of three antioxidant enzymes: superoxide dismutase 1 (SOD1), glutathione reductase (Glu-Red), and biliverdin reductase B (BLVRB). SOD1 converts superoxide radicals into less harmful hydrogen peroxide and molecular oxygen. Glutathione reductase (Glu-Red) plays a crucial role in maintaining the levels of reduced glutathione, a vital antioxidant molecule in the cell. Biliverdin reductase B (BLVRB) is involved in the conversion of biliverdin into bilirubin, which possesses potent antioxidant properties and can help quench reactive oxygen species (Baranano et al. 2002; Sedlak and Snyder 2004).
All tested concentrations decreased protein expression of SOD1 in human beta-cells: 1 μM TDCIPP to 79% of control, 10 μM TDCIPP to 71% of control, 1 μM TPhP to 76% of control, and 10 μM TPhP to 70% of control (Fig. 8D). In rat beta-cells, SOD1 protein expression remained unchanged (Fig. 8A).
The protein expression of (A, D) superoxide dismutase 1 (SOD1), (B, E) glutathione reductase (Glu-Red), and (C, F) biliverdin reductase B (BLVRB) in the rat (INS1E) pancreatic beta-cells and human (NES2Y) pancreatic beta-cells exposed to 1 μM and 10 μM TDCIPP and TPhP for 1 week. Actin or HPRT was used as a loading control. The graphs represent an average of densitometric analyses of at least four western blots made of four independent sets of samples ± SEM. Below each graph, a representative western blot is shown. *means statistical significance (p < 0.05), **means statistical significance (p < 0.01), determined by ONE-WAYANOVA (Dunnet’s test)
In human beta-cells, protein expression of glutathione reductase (Glu-Red) remained unchanged (Fig. 8E). In rat beta-cells, 10 μM TDCIPP significantly decreased Glu-Red expression to 60% of the control and 10 μM TPhP to 49% of the control (Fig. 8B).
None of the tested concentrations altered BLVRB (biliverdin reductase B) protein expression in beta-cells (Fig. 8C and 8F).
Discussion
Our findings show that the exposure to TDCIPP and TPhP altered intracellular insulin levels and had an impact on other pancreatic beta-cell functions and homeostasis in both rat (INS1E) and human (NESY2) cell lines at non-cytotoxic concentrations (Table 1). More specifically, NADPH production, oxidative stress, glucose transport, ER stress, and lipid metabolism were impacted. These findings are in line with a few previous in vivo studies that reported the effects of TDCIPP and TPhP (in a mixture with tricresyl phosphate) on insulin levels and glucose clearance (Krumm et al. 2018; Walley et al. 2021). Altogether, these results raise the concern that exposure to these flame retardants could promote diabetes or obesity, calling for providing additional evidence.
Although both chemicals impacted pancreatic beta-cell lines, substantial differences existed between the effects of TDCIPP vs. TPhP, especially in rat beta-cells, with opposite effects on insulin production. In addition, differences existed between how each chemical affected INS1E and NESY2 cells. That may reflect a fundamental difference between rat and human species, highlighting the importance of including cell lines from different species when in vitro testing pancreatic beta-cell lines. These differences might also reveal disparities between cell lines with glucose-sensitive vs. constitutive insulin secretion. For example, the TDCIPP-induced increase in p-IRE1α was observed in rat beta-cells with glucose-stimulated insulin expression but not in human beta-cells with constitutive insulin secretion. In fact, the higher protein synthesis rate in cells with glucose-stimulated insulin expression might lead to a faster accumulation of unfolded proteins, making them more prone to ER stress than cells with constitutive insulin secretion. In the future, using glucose-sensitive human cell lines would be important. However, while glucose-sensitive rodent beta-cell lines exist (e.g., INS1E, INS/832, MIN6), establishing a glucose-sensitive human beta-cell line has been more challenging. The EndoC-betaH cell line is the only human glucose-sensitive beta-cell line available. Nevertheless, their cost, ease of use, and unfitness for long-term experiments limit their use.
Metabolites of both FRs are detected at high frequencies in human biomonitoring studies (Chen et al. 2018; Saillenfait et al. 2018; van der Schyff et al. 2023). The estimated daily intakes (EDIs) from levels of metabolites in urines or indoor dust ingestion range from 0.01 to 1560 ng/kg/day, with the highest values in children derived from studies in China and Latvia (Demirtepe et al. 2019; Ding et al. 2021; He et al. 2016; Chen et al. 2018; Li et al. 2019; Pasecnaja et al. 2021; Plichta et al. 2022). Estimating the human equivalent doses (HEDs) corresponding to the in vitro concentrations used in this study requires quantitative in vitro-to-in vivo extrapolation (Najjar et al. 2022). A recent study using such an approach calculated that 5 µM effective doses in in-vitro hepatic cell models would correspond to approximately 0.2 (for TDCIPP) and 0.6 (for TPhP) mg/kg/d in children, which are several orders of magnitude higher than the human EDIs (Ding et al. 2021). On the other hand, the OPFRs plasma concentrations in µM estimated from indoor dust using a high-throughput toxicokinetic model range from 0.003 to 32 µM for TDCIPP and from 0.00006 to 1.4 µM for TPhP (Blum et al. 2019a). Therefore, the concentrations used in our study would be in the range of the highest estimated plasma concentrations. However, caution should be taken when comparing the nominal concentrations used in vitro with real-life exposures since it relies on model-based estimates, and other factors may also influence. For instance, the concentration of free chemicals in the medium may differ from the nominal concentration due, for example, to sorption to components of the in vitro setup (e.g., plastic and serum) (Groothuis et al. 2019). In addition, in our study, the exposure is limited to 1 week while the human population is potentially exposed chronically to these flame retardants.
Effect of TDCIPP and TPhP in rat beta-cells
TDCIPP decreased the intracellular insulin and proinsulin levels in the rat beta-cell line. The TDCIPP-induced increase in ER stress might contribute, at least partially, to the decrease in insulin production observed following exposure to TDCIPP. Indeed, ER stress in pancreatic beta-cells leads to a sudden drop in insulin production and the manifestation of type 2 diabetes mellitus (Burgos-Morón et al. 2019; Morikawa and Urano 2023).
However, in cells exposed to 10 μM TDCIPP, the reduced intracellular insulin levels did not lead to a decrease in secreted insulin levels at the time examined. In our study, we detected effects on several proteins that might increase insulin secretion, compensating for the decrease in insulin production and normalizing the levels of secreted insulin. For instance, the increase in IDH1 and the decrease in Glu-Red may elevate the levels of NADPH. Indeed, IDH1 inhibition has been shown to suppress insulin secretion in wild-type mouse islets (Bauchle et al. 2021). Glu-Red is highly expressed in pancreatic islet cells (Nagaoka et al. 2004), where it produces reduced glutathione needed for oxidative stress defense but also consumes NADPH needed for the amplification of insulin secretion. Its expression (Guo et al. 2013) and activity (Xiao et al. 2023) were decreased in the diabetic mice pancreases and MIN6 mouse beta-cells exposed to a high glucose concentration (Xu et al. 2023). In addition, the decreasing trend of aconitase-2 expression, albeit statistically insignificant, may contribute to the overall impact on NADPH production by reducing the substrate level for IDH1. Another potential mechanism involves the increased expression of ATP-CL that may stimulate insulin secretion through malonyl-CoA production (Aghelan et al. 2020). Indeed, Flamez and coworkers showed that ATP-CL inhibitors radicicol and (-)-hydroxy-citrate blocked part of glucose-stimulated insulin secretion in rat beta-cells (Flamez et al. 2002). Therefore, the elevated levels of IDH1, the reduction of Glu-Red levels, and the increased expression of ATP-CL in rat beta-cells exposed to 10 μM TDCIPP may work in concert to normalize insulin secretion despite the decrease in intracellular insulin levels. In such a scenario, longer-term or chronic exposures to TDCIPP might decrease the levels of secreted insulin after the levels of intracellular insulin are too low or depleted.
Contrary to TDCIPP, TPhP exposure increased intracellular proinsulin and insulin levels in INS1E cells. We did not detect significant changes in the levels of secreted insulin in cells exposed to 10 μM TPhP, but an increasing trend was consistent with the effect on insulin production. The decreased levels of Glu-Red might potentially contribute to an increase in insulin secretion by elevating the levels of NADPH. Excessive insulin production can lead to a more rapid decline in blood glucose levels, triggering hunger. Consequently, compounds exhibiting such effects have the potential to promote obesity. Obesity as a result of hyperinsulinemia was previously described, e.g., in mice overproducing glutathione peroxidase 1 (Wang et al. 2008). Interestingly, unlike in macrophages and hepatocytes (Hu et al. 2020; Yue et al. 2023), TPhP failed to induce ER stress in beta-cells, suggesting differential effects on ER stress induction in various cell types. The exposure to 10 μM TPhP also decreased PLIN5 expression in rat beta-cells. PLIN5 plays a dual role in lipid metabolism by participating in lipid droplet formation while, under certain conditions, also promoting lipolysis and fatty acid oxidation to meet metabolic demands (Zhu et al. 2019). Moreover, in INS1E cells, overexpression of PLIN5 has been shown to protect against palmitic acid-induced toxicity (Zhu et al. 2019). This protective effect is attributed to PLIN5’s ability to decrease endoplasmic reticulum (ER) stress (Zhu et al. 2019) and increase the expression of Nrf2, a key regulator of cellular antioxidant response (Zhu et al. 2020). By reducing PLIN5 expression, TPhP may disrupt the balance of lipid metabolism and impair beta-cells’ ability to handle excessive fatty acids, increasing their susceptibility to lipotoxic damage. Several studies have described the effects of TPhP exposure on fat metabolism. 10 μM TPhP increased lipid accumulation in liver cells (Negi et al. 2021), adipogenic differentiation and lipolysis in 3T3-L1 adipocytes (Cano-Sancho et al. 2017) and induced total cholesterol and total triglyceride accumulation in HepG2 cells (Hao et al. 2019). However, it is important to note that lipid droplet formation likely has a beneficial function in beta-cells (Sramek et al. 2021). In the case of fatty acid overload, beta-cells utilize lipid droplets to sequester fatty acids, preventing them from inducing toxicity (Tong et al. 2022; Zheng et al. 2022).
Effect of TDCIPP and TPhP in human beta-cells
The effects on insulin production and secretion could not be measured in the human beta-cell line, but TDCIPP and TPhP impacted glucose transporters, IDH1, ATF3, and SOD1. In addition, both TDCIPP and TPhP increased GLUT1 expression while decreasing GLUT2 expression. In rodent beta-cells, the primary glucose transporter responsible for glucose uptake into the cells is GLUT2 (Mueckler and Thorens 2013). Interestingly, the islets isolated from GLUT2-null mice demonstrated impaired glucose-stimulated insulin secretion and synthesis, highlighting the significance of GLUT2 in these processes (Guillam et al. 2000). Although similar experiments in human pancreatic beta-cells are not available, research by McCulloch et al. suggests that GLUT1, rather than GLUT2, is primarily responsible for transporting glucose into human beta-cells (McCulloch et al. 2011). Therefore, the increase in GLUT1 levels might have a more significant impact than the decrease in GLUT2 and it is plausible to expect an overall increase in glucose transport in human beta-cells exposed to TDCIPP or TPhP.
In human beta-cells, all four exposure conditions significantly decreased SOD1 levels. The decreased SOD1 levels could increase the vulnerability of pancreatic beta-cells against superoxide. Interestingly, SOD1-knockout mice showed decreased insulin secretion (Muscogiuri et al. 2013). Together with the decrease in IDH1, the decrease in SOD1 might, therefore, contribute to reduced insulin secretion in human cells.
The exposure to 10 μM TCDIPP and 10 μM TPhP also negatively affected ATF3 levels in human beta-cells. Studies in rodents suggest that ATF3 is required for proper insulin production in response to a fatty diet, having a rather protective effect on pancreatic beta-cells (Zmuda et al. 2010). When exposed to a high-fat diet, mice with a knockout ATF3 gene had significantly reduced serum insulin levels compared to wild-type mice (Zmuda et al. 2010). In human beta-cells, TDCIPP- and TPhP-induced decrease in ATF3 levels may potentially diminish the defense mechanisms of these cells against various adverse conditions. However, further research is needed to fully understand the role of ATF3 in stress defense and its implications for human beta-cell function and insulin secretion.
TPhP also impacted perilipins in human cell lines, decreasing PLIN5 expression while increasing PLIN2. PLIN2, also known as adipose differentiation-related protein (ADRP), is primarily associated with lipid droplets and is expressed in various tissues, including pancreatic beta-cells (Tong et al. 2022). However, its overexpression in beta-cells appears to have negative implications. Research conducted by Chen and coworkers (Chen et al. 2017) demonstrated that in mouse islets and the mouse beta-cell line MIN6, overexpression of PLIN2 led to increased endoplasmic reticulum (ER) stress when exposed to lipid overload or chemical ER stress inducers. Conversely, silencing of PLIN2 alleviated this effect, suggesting a detrimental role of PLIN2 in promoting ER stress (Chen et al. 2017). Additionally, elevated expression of PLIN2 was observed in human islets obtained from donors with type 2 diabetes mellitus (Tong et al. 2022). The overexpression of PLIN2 in human beta-cells exposed to 10 μM TPhP likely contributes together with PLIN5 downregulation to an imbalance in lipid handling and storage within beta-cells, potentially leading to disturbances in lipid homeostasis. This disruption in lipid balance can have detrimental consequences for beta-cell function and insulin secretion when facing saturated fatty acids or nutritional overload.
Conclusions
Our findings indicate that the exposure to TDCIPP and TPhP impacted pancreatic beta-cell functions and homeostasis in both rat (INS1E) and human (NESY2) cell lines. Notably, both chemicals altered intracellular insulin and proinsulin levels in rat cells. Although we did not observe significant changes in the secreted insulin levels, we theorize that more extended exposure periods exceeding one week may eventually impact insulin secretion. Insulin levels could not be measured in human cells, but the effects on NADPH production, glucose transport, or oxidative stress in human and rat cells might alter insulin production and secretion. The alterations in protein expression, as shown in Table 1, indicate that exposure to flame retardants negatively disrupted beta-cell homeostasis, potentially rendering the beta-cells more susceptible to adverse conditions such as excessive nutrient intake or oxidative stress.
We compared the effects of flame retardants on two different beta-cell lines, rat and human. In most cases, TDCIPP and TPhP induced significant perturbations to both beta-cell lines but with substantial differences in the proteins/pathways affected. That might reflect important differences between rat and human beta-cells, as described before (Benner et al. 2014; Klemen et al. 2017; Law et al. 2010; McCulloch et al. 2011) and/or between cells with glucose-sensitive and constitutive insulin secretion. That highlights the relevance of using cells from both species, ideally glucose-sensitive, when testing chemicals.
Currently, there is a lack of systematic testing for the potential pro-diabetic effects of industrial chemicals before they are introduced to the market. Our results highlight the importance of focusing more on pancreatic cells and diabetes in toxicological studies. Given the ongoing diabetes epidemic, it would greatly benefit public health to develop an in vitro-based test battery that assesses the toxicity of chemicals on pancreatic beta-cells prior to widespread exposure in the population.
Data Availability
The complete data supporting the findings present in the article can be provided by the corresponding author upon a reasonable request.
References
Aghelan Z, Kiani S, Nasiri A, Sadeghi M, Farrokhi A, Khodarahmi R (2020) Factors influencing mitochondrial function as a key mediator of glucose-induced insulin release: highlighting nicotinamide nucleotide transhydrogenase. Int J Mol Cell Med 9:107–122
Bailey MJ, Naik NN, Wild LE, Patterson WB, Alderete TL (2020) Exposure to air pollutants and the gut microbiota: a potential link between exposure, obesity, and type 2 diabetes. Gut Microbes 11:1188–1202
Bajard L, Melymuk L, Blaha L (2019) Prioritization of hazards of novel flame retardants using the mechanistic toxicology information from ToxCast and adverse outcome pathways. Environ Sci Eur 31:19
Bajard L, Negi CK, Mustieles V, Melymuk L, Jomini S, Barthelemy-Berneron J, Fernandez MF, Blaha L (2021) Endocrine disrupting potential of replacement flame retardants-Review of current knowledge for nuclear receptors associated with reproductive outcomes. Environ Int 153:12
Baranano DE, Rao M, Ferris CD, Snyder SH (2002) Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A 99:16093–16098
Bauchle CJ, Rohli KE, Boyer CK, Pal V, Rocheleau JV, Liu SM, Imai Y, Taylor EB, Stephens SB (2021) Mitochondrial efflux of citrate and isocitrate is fully dispensable for glucose-stimulated insulin secretion and pancreatic islet beta-cell function. Diabetes 70:1717–1728
Benner C, van der Meulen T, Caceres E, Tigyi K, Donaldson CJ, Huising MO (2014) The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics 15:16
Blum A, Behl M, Birnbaum L, Diamond ML, Phillips A, Singla V, Sipes NS, Stapleton HM, Venier M (2019a) Organophosphate ester flame retardants: are they a regrettable substitution for polybrominated diphenyl ethers? Environ Sci Technol Lett 6(11):638–649
Blum A, Behl M, Birnbaum LS, Diamond ML, Phillips A, Singla V, Sipes NS, Stapleton HM, Venier M (2019b) Organophosphate ester flame retardants: are they a regrettable substitution for polybrominated diphenyl ethers? Environ Sci Technol Lett 6:638–649
Bresson SE, Isom S, Jensen ET, Huber S, Oulhote Y, Rigdon J, Lovato J, Liese AD, Pihoker C, Dabelea D, Ehrlich S, Ruzzin J (2022) Associations between persistent organic pollutants and type 1 diabetes in youth. Environ Int 163:11
Burgos-Morón E, Abad-Jiménez Z, de Marañón AM, Iannantuoni F, Escribano-López I, López-Domènech S, Salom C, Jover A, Mora V, Roldan I, Solá E, Rocha M, Víctor VM (2019) Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. J Clin Med 8:22
Campbell JE, Newgard CB (2021) Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol 22:142–158
Cano-Sancho G, Smith A, La Merrill MA (2017) Triphenyl phosphate enhances adipogenic differentiation, glucose uptake and lipolysis via endocrine and noradrenergic mechanisms. Toxicol Vitro 40:280–288
Chen E, Tsai TH, Li L, Saha P, Chan L, Chang BHJ (2017) PLIN2 is a key regulator of the unfolded protein response and endoplasmic reticulum stress resolution in pancreatic beta cells. Sci Rep 7:12
Chen Y, Fang J, Ren L, Fan R, Zhang J, Liu G, Zhou L, Chen D, Yu Y, Lu S (2018) Urinary metabolites of organophosphate esters in children in South China: concentrations, profiles and estimated daily intake. Environ Pollut. https://doi.org/10.1016/j.envpol.2017.12.092
Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P (2017) Endoplasmic reticulum stress and eIF2alpha phosphorylation: The Achilles heel of pancreatic beta cells. Mol Metab 6:1024–1039
Demirtepe H, Melymuk L, Diamond ML, Bajard L, Vojta S, Prokes R, Sanka O, Klanova J, Palkovicova Murinova L, Richterova D, Rasplova V, Trnovec T (2019) Linking past uses of legacy SVOCs with today’s indoor levels and human exposure. Environ Int 127:653–663
Ding J, Liu W, Zhang H, Zhu L, Zhu L, Feng J (2021) Liver-based probabilistic risk assessment of exposure to organophosphate esters via dust ingestion using a physiologically based toxicokinetic (PBTK) model. Int J Environ Res Public Health 18(23):12496
Dishaw LV, Macaulay LJ, Roberts SC, Stapleton HM (2014) Exposures, mechanisms, and impacts of endocrine-active flame retardants. Curr Opin Pharmacol 19:125–133
Ferdaoussi M, Dai X, Jensen MV, Wang R, Peterson BS, Huang C, Ilkayeva O, Smith N, Miller N, Hajmrle C, Spigelman AF, Wright RC, Plummer G, Suzuki K, Mackay JP, van de Bunt M, Gloyn AL, Ryan TE, Norquay LD, Brosnan MJ, Trimmer JK, Rolph TP, Kibbey RG, Manning Fox JE, Colmers WF, Shirihai OS, Neufer PD, Yeh ET, Newgard CB, MacDonald PE (2015) Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J Clin Invest 125:3847–3860
Flamez D, Berger V, Kruhoffer M, Orntoft T, Pipeleers D, Schuit FC (2002) Critical role for cataplerosis via citrate in glucose-regulated insulin release. Diabetes 51:2018–2024
Fonseca SG, Urano F, Burcin M, Gromada J (2010) Stress hypERactivation in the β-cell. Islets 2:1–9
Gómez-Lechón MJ, Tolosa L, MT, D., (2014) Cell-based models to predict human hepatotoxicity of drugs. Rev Toxicol 31:149–156
Gong J, Wang XZ, Wang T, Chen JJ, Xie XY, Hu H, Yu F, Liu HL, Jiang XY, Fan HD (2017) Molecular signal networks and regulating mechanisms of the unfolded protein response. J Zhejiang Univ-SCI B. 18(1):1–14
Groothuis FA, Timmer N, Opsahl E, Nicol B, Droge STJ, Blaauboer BJ, Kramer NI (2019) Influence of in vitro assay setup on the apparent cytotoxic potency of benzalkonium chlorides. Chem Res Toxicol 32:1103–1114
Guillam MT, Dupraz P, Thorens B (2000) Glucose uptake, utilization, and signaling in GLUT2-null islets. Diabetes 49:1485–1491
Guo C, Li R, Zheng N, Xu L, Liang T, He Q (2013) Anti-diabetic effect of ramulus mori polysaccharides, isolated from Morus alba L., on STZ-diabetic mice through blocking inflammatory response and attenuating oxidative stress. Int Immunopharmacol 16:93–99
Hao ZL, Zhang ZJ, Lu DZ, Ding B, Shu L, Zhang Q, Wang C (2019) Organophosphorus flame retardants impair intracellular lipid metabolic function in human hepatocellular cells. Chem Res Toxicol 32:1250–1258
He R, Li Y, Xiang P, Li C, Zhou C, Zhang S, Cui X, Ma LQ (2016) Organophosphorus flame retardants and phthalate esters in indoor dust from different microenvironments: bioaccessibility and risk assessment. Chemosphere 150:528–535
Hoffmann S, Kleinstreuer N, Alepee N, Allen D, Api AM, Ashikaga T, Clouet E, Cluzel M, Desprez B, Gellatly N, Goebel C, Kern PS, Klaric M, Kuhnl J, Lalko JF, Martinozzi-Teissier S, Mewes K, Miyazawa M, Parakhia R, van Vliet E, Zang Q, Petersohn D (2018) Non-animal methods to predict skin sensitization (I): the Cosmetics Europe database<sup/>. Crit Rev Toxicol 48:344–358
Hoyeck MP, Matteo G, MacFarlane EM, Perera I, Bruin JE (2022) Persistent organic pollutants and beta-cell toxicity: a comprehensive review. Am J Physiol Endocrinol Metab 322:E383–E413
Hu WX, Kang QY, Zhang CH, Ma HJ, Xu CK, Wan Y, Hu JY (2020) Triphenyl phosphate modulated saturation of phospholipids: Induction of endoplasmic reticulum stress and inflammation. Environ Pollut 263:7
Jiang CX, Wang YP, Guo M, Long Y, Chen J, Fan F, Tang S, Xu Y (2021) PCB118 induces inflammation of islet beta cells via activating ROS-NLRP3 inflammasome signaling. Biomed Res Int 2021:8
Kim YA, Kim HY, Oh YJ, Kwon WY, Lee MH, Bae JY, Woo MS, Kim JM, Yoo YH (2018) Polychlorinated biphenyl 138 exposure-mediated lipid droplet enlargement endows adipocytes with resistance to TNF-alpha-induced cell death. Toxicol Lett 292:55–62
Kim YA, Park JB, Woo MS, Lee SY, Kim HY, Yoo YH (2019) Persistent organic pollutant-mediated insulin resistance. Int J Environ Res Public Health 16:14
Kleinstreuer NC, Hoffmann S, Alepee N, Allen D, Ashikaga T, Casey W, Clouet E, Cluzel M, Desprez B, Gellatly N, Gobel C, Kern PS, Klaric M, Kuhnl J, Martinozzi-Teissier S, Mewes K, Miyazawa M, Strickland J, van Vliet E, Zang Q, Petersohn D (2018) Non-animal methods to predict skin sensitization (II): an assessment of defined approaches. Crit Rev Toxicol 48:359–374
Klemen MS, Dolensek J, Rupnik MS, Stozer A (2017) The triggering pathway to insulin secretion: functional similarities and differences between the human and the mouse cells and their translational relevance. Islets 9:109–139
Krewski D, Acosta D Jr, Andersen M, Anderson H, Bailar JC 3rd, Boekelheide K, Brent R, Charnley G, Cheung VG, Green S Jr, Kelsey KT, Kerkvliet NI, Li AA, McCray L, Meyer O, Patterson RD, Pennie W, Scala RA, Solomon GM, Stephens M, Yager J, Zeise L (2010) Toxicity testing in the 21st century: a vision and a strategy. J Toxicol Environ Health B Crit Rev 13:51–138
Krumm EA, Patel VJ, Tillery TS, Yasrebi A, Shen JL, Guo GL, Marco SM, Buckley BT, Roepke TA (2018) Organophosphate flame-retardants alter adult mouse homeostasis and gene expression in a sex-dependent manner potentially through interactions with ERα. Toxicol Sci 162:212–224
Ku HC, Cheng CF (2020) Master regulator activating transcription factor 3 (ATF3) in metabolic homeostasis and cancer. Front Endocrinol 11:556
Langley G, Evans T, Holgate ST, Jones A (2007) Replacing animal experiments: choices, chances and challenges. BioEssays 29:918–926
Law E, Lu S, Kieffer TJ, Warnock GL, Ao Z, Woo M, Marzban L (2010) Differences between amyloid toxicity in alpha and beta cells in human and mouse islets and the role of caspase-3. Diabetologia 53:1415–1427
Lee YM, Jacobs DR, Lee DH (2018) Persistent organic pollutants and type 2 diabetes: a critical review of review articles. Front Endocrinol 9:9
Lenzen S (2017) Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic beta-cells. Biochim Biophys Acta-Gen Subj 1861:1929–1942
Li W, Wang Y, Asimakopoulos AG, Covaci A, Gevao B, Johnson-Restrepo B, Kumosani TA, Malarvannan G, Moon HB, Nakata H, Sinha RK, Tran TM, Kannan K (2019) Organophosphate esters in indoor dust from 12 countries: concentrations, composition profiles, and human exposure. Environ Int. https://doi.org/10.1016/j.envint.2019.105178
Loiola RA, dos Anjos FM, Shimada AL, Cruz WS, Drewes CC, Rodrigues SF, Cardozo KHM, Carvalho VM, Pinto E, Farsky SH (2016) Long-term <i>in vivo</i> polychlorinated biphenyl 126 exposure induces oxidative stress and alters proteomic profile on islets of Langerhans. Sci Rep 6:12
Marroqui L, Tudurí E, Alonso-Magdalena P, Quesada I, Nadal A, dos Santos RS (2018) Mitochondria as target of endocrine-disrupting chemicals: implications for type 2 diabetes. J Endocrinol 239:R27–R45
McCulloch LJ, van de Bunt M, Braun M, Frayn KN, Clark A, Gloyn AL (2011) GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Mol Genet Metab 104:648–653
Morikawa S, Urano F (2023) The role of ER stress in diabetes: exploring pathological mechanisms using wolfram syndrome. Int J Mol Sci 24:13
Mueckler M, Thorens B (2013) The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med 34:121–138
Mullerova D, Pesta M, Cedikova M, Dvorakova J, Kulda V, Srbecka K, Muller L, Dvorak P, Kripnerova M, Kralickova M, Babuska V, Kuncova J (2016) DDE downregulates PLIN2 expression during differentiation of mesenchymal stem cells into adipocytes in lipid-enriched medium. J Appl Biomed 14:113–117
Muscogiuri G, Salmon AB, Aguayo-Mazzucato C, Li M, Balas B, Guardado-Mendoza R, Giaccari A, Reddick RL, Reyna SM, Weir G, Defronzo RA, Van Remmen H, Musi N (2013) Genetic disruption of SOD1 gene causes glucose intolerance and impairs beta-cell function. Diabetes 62:4201–4207
Nagaoka Y, Iuchi Y, Ikeda Y, Fujii J (2004) Glutathione reductase is expressed at high levels in pancreatic islet cells. Redox Rep 9:321–324
Najjar A, Punt A, Wambaugh J, Paini A, Ellison C, Fragki S, Bianchi E, Zhang F, Westerhout J, Mueller D, Li H, Shi Q, Gant TW, Botham P, Bars R, Piersma A, van Ravenzwaay B, Kramer NI (2022) Towards best use and regulatory acceptance of generic physiologically based kinetic (PBK) models for in vitro-to-in vivo extrapolation (IVIVE) in chemical risk assessment. Arch Toxicol 96(12):3407–3419
Negi CK, Bajard L, Kohoutek J, Blaha L (2021) An adverse outcome pathway based in vitro characterization of novel flame retardants-induced hepatic steatosis. Environ Pollut 289:11
Park CM, Kim KT, Rhyu DY (2020) Low-concentration exposure to organochlorine pesticides (OCPs) in L6 myotubes and RIN-m5F pancreatic beta cells induces disorders of glucose metabolism. Toxicol in Vitro 65:104767
Pasecnaja E, Perkons I, Bartkevics V, Zacs D (2021) Legacy and alternative brominated, chlorinated, and organophosphorus flame retardants in indoor dust-levels, composition profiles, and human exposure in Latvia. Environ Sci Pollut Res 28(20):25493–25502
Pavlikova N, Smetana P, Halada P, Kovar J (2015) Effect of prolonged exposure to sublethal concentrations of DDT and DDE on protein expression in human pancreatic beta cells. Environ Res 142:257–263
Pavlikova N, Daniel P, Sramek J, Jelinek M, Sramkova V, Nemcova V, Balusikova K, Halada P, Kovar J (2019) Upregulation of vitamin D-binding protein is associated with changes in insulin production in pancreatic beta-cells exposed to p, p’-DDT and p, p’-DDE. Sci Rep 9:18026
Pavlikova N, Sramek J, Jacek M, Kovar J, Nemcova V (2023) Targets for pollutants in rat and human pancreatic beta-cells: The effect of prolonged exposure to sub-lethal concentrations of hexachlorocyclohexane isomers on the expression of function- and survival-related proteins. Environ Toxicol Pharmacol 104:104299
Plichta V, Steinwider J, Vogel N, Weber T, Kolossa-Gehring M, Murinova LP, Wimmerova S, Tratnik JS, Horvat M, Koppen G, Govarts E, Gilles L, Rodriguez Martin L, Schoeters G, Covaci A, Fillol C, Rambaud L, Jensen TK, Rauscher-Gabernig E (2022) Risk assessment of dietary exposure to organophosphorus flame retardants in children by using HBM-Data. Toxics 10(5):234
Pratt EPS, Harvey KE, Salyer AE, Hockerman GH (2019) Regulation of cAMP accumulation and activity by distinct phosphodiesterase subtypes in INS-1 cells and human pancreatic beta-cells. PLoS ONE 14:e0215188
Saillenfait AM, Ndaw S, Robert A, Sabate JP (2018) Recent biomonitoring reports on phosphate ester flame retardants: a short review. Archives Toxicol 92:2749–2778
Sedlak TW, Snyder SH (2004) Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics 113:1776–1782
Sramek J, Nemcova-Furstova V, Kovar J (2021) Molecular mechanisms of apoptosis induction and its regulation by fatty acids in pancreatic beta-cells. Int J Mol Sci 22(8):4285
Tinant G, Neefs I, Das K, Rees JF, Larondelle Y, Debier C (2021) Methylmercury displays pro-adipogenic properties in rainbow trout preadipocytes. Chemosphere 263:11
Tong X, Liu SM, Stein R, Imai Y (2022) Lipid droplets’ role in the regulation of beta-cell function and beta-cell demise in type 2 diabetes. Endocrinology 163:11
van der Schyff V, Kalina J, Govarts E, Gilles L, Schoeters G, Castano A, Esteban-Lopez M, Kohoutek J, Kukucka P, Covaci A, Koppen G, Andryskova L, Piler P, Klanova J, Jensen TK, Rambaud L, Riou M, Lamoree M, Kolossa-Gehring M, Vogel N, Weber T, Goen T, Gabriel C, Sarigiannis DA, Sakhi AK, Haug LS, Murinova LP, Fabelova L, Tratnik JS, Mazej D, Melymuk L (2023) Exposure to flame retardants in European children - results from the HBM4EU aligned studies. Int J Hygiene Environ Health. https://doi.org/10.1016/j.ijheh.2022.114070
Walley SN, Krumm EA, Yasrebi A, Kwiecinski J, Wright V, Baker C, Roepke TA (2021) Maternal organophosphate flame-retardant exposure alters offspring energy and glucose homeostasis in a sexually dimorphic manner in mice. J Appl Toxicol 41:572–586
Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG (2008) Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia 51:1515–1524
Wang J, Yang X, Zhang JJ (2016) Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic β cells. Cell Signal 28:1099–1104
Wang D, Yan S, Yan J, Teng M, Meng Z, Li R, Zhou Z, Zhu W (2019) Effects of triphenyl phosphate exposure during fetal development on obesity and metabolic dysfunctions in adult mice: Impaired lipid metabolism and intestinal dysbiosis. Environ Pollut 246:630–638
Xiao X, Erukainure OL, Islam MS (2023) Red honeybush tea mitigates oxidative imbalance and hyperlipidemia, while improving glucose homeostasis in type 2 diabetic rats. Food Sci Hum Well 12:2029–2039
Xu J, Fan X, Zhu M, Zhao Z, Qi X, Gao H, Liu Y, Shi Y, Zhang C (2023) Ginsenoside Rg3 protects mouse islet beta-cells injured by high glucose. Indian J Microbiol 63:173–180
Yang BY, Fan SJ, Thiering E, Seissler J, Nowak D, Dong GH, Heinrich J (2020) Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ Res 180:11
Yao X, Geng S, Zhu L, Jiang H, Wen J (2023) Environmental pollutants exposure and gestational diabetes mellitus: evidence from epidemiological and experimental studies. Chemosphere 332:138866
Yue J, Sun X, Duan X, Sun C, Chen H, Sun H, Zhang L (2023) Triphenyl phosphate proved more potent than its metabolite diphenyl phosphate in inducing hepatic insulin resistance through endoplasmic reticulum stress. Environ Int 172:107749
Zhang GF, Jensen MV, Gray SM, El K, Wang Y, Lu DH, Becker TC, Campbell JE, Newgard CB (2021) Reductive TCA cycle metabolism fuels glutamine- and glucose-stimulated insulin secretion. Cell Metab 33(4):804-817.e5
Zhao JY, Zhan ZX, Lu MJ, Tao FB, Wu D, Gao H (2022) A systematic scoping review of epidemiological studies on the association between organophosphate flame retardants and neurotoxicity. Ecotoxicol Environ Saf 243:113973
Zheng XF, Ho QWC, Chua M, Stelmashenko O, Yeo XY, Muralidharan S, Torta F, Chew EGY, Lian MM, Foo JN, Jung S, Wong SH, Tan NS, Tong NW, Rutter GA, Wenk MR, Silver DL, Berggren PO, Ali Y (2022) Destabilization of beta Cell FIT2 by saturated fatty acids alter lipid droplet numbers and contribute to ER stress and diabetes. Proc Natl Acad Sci U S A 119:10
Zhu YX, Zhang XY, Zhang L, Zhang ML, Li L, Luo D, Zhong Y (2019) Perilipin5 protects against lipotoxicity and alleviates endoplasmic reticulum stress in pancreatic beta-cells. Nutr Metab 16:14
Zhu YX, Ren CX, Zhang ML, Zhong Y (2020) Perilipin 5 reduces oxidative damage associated with lipotoxicity by activating the PI3K/ERK-mediated Nrf2-ARE signaling pathway in INS-1 pancreatic beta-cells. Front Endocrinol 11:14
Zmuda EJ, Qi L, Zhu MX, Mirmira RG, Montminy MR, Hai T (2010) The Roles of ATF3, an adaptive-response gene, in high-fat-diet-induced diabetes and pancreatic beta-cell dysfunction. Mol Endocrinol 24:1423–1433
Acknowledgements
This work has been supported by the Charles University Cooperatio METD, the project National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104)—Funded by the European Union—Next Generation EU., and the Research Infrastructure RECETOX RI (No LM2023069) financed by the Ministry of Education, Youth and Sports.
Funding
Open access publishing supported by the National Technical Library in Prague. Univerzita Karlova v Praze,Cooperatio METD.
Author information
Authors and Affiliations
Contributions
Nela Pavlikova contributed to conceptualization, resources, investigation, formal analysis, and writing. Jan Sramek and Vlasta Nemcova contributed to the investigation and editing. Lola Bajard contributed to conceptualization, writing, and visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pavlíková, N., Šrámek, J., Němcová, V. et al. Effects of novel flame retardants tris(1,3-dichloro-2-propyl) phosphate (TDCIPP) and triphenyl phosphate (TPhP) on function and homeostasis in human and rat pancreatic beta-cell lines. Arch Toxicol (2024). https://doi.org/10.1007/s00204-024-03841-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00204-024-03841-z