Abstract
Idiosyncratic drug-induced liver injury is a rare and unpredictable event. Deciphering its initiating-mechanism is a hard task as its occurrence is individual dependent. Thus, studies that utilize models that are not individual-centric might drive to a general mechanistic conclusion that is not necessarily true. Here, we use the individual-centric spheroid model to analyze the initiating-mechanism of troglitazone-mediated iDILI risk. Individual-centric spheroid models were generated using a proprietary cell educating technology. These educated spheroids contain hepatocytes, hepatic stellate cells, activated monocyte-derived macrophages, and dendritic cells under physiological conditions. We show that phases 1 and 2 drug-metabolizing enzymes were induced in an individual-dependent manner. However, we did not observe any association of DEMs induction and troglitazone (TGZ)-mediated iDILI risk. We analyzed TGZ-mediated iDILI and found that a 44-year-old male showed iDILI risk that is associated with TGZ-mediated suppression of IL-12 expression by autologous macrophages and dendritic cells. We performed a rescue experiment and showed that treatment of spheroids from this 44-year-old male with TGZ and recombinant IL-12 suppressed iDILI risk. We confirmed the mechanism in another 31-year-old female with iDILI risk. We demonstrate here that individual-centric spheroid are versatile models that allow to predict iDILI risk and to analyze a direct effect of the drug on activated macrophages and dendritic cells to uncover the initiating-mechanism of iDILI occurrence. This model opens perspectives for a personalized strategy to mitigate iDILI risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiosyncratic drug-induced liver injury (iDILI) is a major issue for patients and for drug development because of its unpredictable nature (Jee et al. 2021). It is a rare event that occurs independently from the dose and from the duration of administration (Fontana et al. 2023). It is generally believed to be immune related, and its occurrence is presumably dependent of the biological characteristics of the individual (Uetrecht 2019; Yamashita et al. 2017). The initiating mechanism of iDILI is challenging to understand because retrospective studies in humans do not allow to separate cause from effect. Moreover, available preclinical models are insufficient for mechanistic studies (Segovia-Zafra et al. 2021). Therefore, uncovering the molecular mechanism of iDILI onset in a subject, is a hard task during drug development as there is a lack of individual-centric preclinical models that recapitulate key biological features.
Macrophages play an important role in the development of iDILI (Mak and Uetrecht 2019). These immune cells are extremely plastic and could assume diverse phenotypes and functions in response to the microenvironment in which they live (Lendeckel et al. 2022). Previous studies have reported that the large interindividual variation of the interaction between hepatocytes and macrophages is responsible for substance specific hepatotoxicity (Cheng et al. 2021; Padberg et al. 2021). Among the immune cells, dendritic cells (DCs) can also participate to iDILI by secreting specific subsets of cytokines (Allison et al. 2023; Mendez-Sanchez et al. 2021). It is, therefore, obvious that a good preclinical model to evaluate iDILI should incorporate autologous macrophages and DCs at physiological conditions. Unfortunately, studies on immune cell functions were conducted so far with differentiated macrophages and DCs that were prepared under non-physiological conditions because host factors and biological environments are not reproduced in vitro(Kenna and Uetrecht 2018; Villar et al. 2023).
Troglitazone (TGZ) is an antidiabetic that was removed from the market in 2000. During its development stage, no liver toxicity was reported. However, once released severe cases of iDILI were observed leading to liver transplant or death (Chojkier 2005; Funk et al. 2001). The molecular mechanism of TGZ-mediated iDILI remains unclear. Several works reported that TGZ upregulates CYP3A4, CYP2B6, and CYP2C9 expression, and it is metabolized into reactive metabolites that cause liver injury. Nevertheless, the role of reactive metabolites in TGZ-mediated iDILI remains controversial as no in vivo nor in vitro study has demonstrated a direct mechanism to explain the hepatotoxicity that develops in some individuals. Some studies have reported that elevated concentrations of troglitazone can induce mitochondrial dysfunction(Haskins et al. 2001; Tirmenstein et al. 2002). However, no measurable mitochondrial dysfunction, oxidant stress, nor liver injury was observed in rodents treated with TGZ(Haskins et al. 2001). These data suggest that despite considerable efforts that have been made to elucidate the mechanism of TGZ-mediated liver injury, no general conclusion can be stressed probably because the mechanism of TGZ-induced iDILI is subject dependent and should be analyzed individually.
We have previously developed individual-centric spheroid models using our proprietary cell educating technology (Cherradi et al. 2023). We demonstrated that our individual-centric models can detect immune-mediated iDILI as they contain hepatic cells and autologous immune cells that were prepared with OneSmartDiff, a tailored cell culture medium (Roux et al. 2024). In the present work, we use these individual-centric spheroid models to uncover the molecular mechanism of TGZ-mediated iDILI in a 44-year-old male. We show that TGZ-mediated induction of phases 1 and 2 drug-metabolizing enzymes (DMEs), and the expression of xenobiotic sensors and transcription factors (TFs) are subject dependent. However, we did not observe any clear relationship between DMEs induction and the risk of iDILI occurrence. Interestingly, we show that TGZ directly repressed immune cells mediated expression of IL-12a transcript in a 44-year-old male with iDILI risk. Finally, we demonstrate that treating spheroids from this 44-year-old male with recombinant IL-12 rescued TGZ-mediated iDILI. We confirm that IL-12 is a driver of TGZ-mediated iDILI in another 31-year-old female. Our data suggest that individual-centric spheroids are powerful preclinical models to uncover individually the mechanistic insights of iDILI.
Materials and methods
Biological samples, cell lines, primary human hepatocytes, and reagents
Patient’s blood was collected and processed by mechanical filtration according to the protocol described elsewhere (Cherradi et al. 2023). Blood samples from healthy donors (> 18-year old, > 50 kg body weight, no viral infection, no chronic disease, and no cancer) were obtained from the Etablissement Français du Sang (EFS) Hauts de France – Normandie. Blood samples from 10 healthy individuals were used to generate educated spheroids to assess iDILI risk of troglitazone.
The research protocol was conducted under French legal guidelines and fulfilled the requirements of the local institutional ethics committee. The study was approved by the “Direction Générale de la recherche et de l’innovation” (CODECOH, n°DC-2021–4779). This project does not involve the human person according to the legislation (article L1121-1 du code de la santé publique). Recombinant human IL-12 was purchased from ACROBiosystems.
Hepatocyte (HepG2) and monocyte (THP-1) lines were from ATCC (Molsheim, France). Hepatic stellate cell line (TWNT-1) was from Glow Biologics (Tarrytown, NY, USA). Cell culture reagents were provided by StemCell (Saint Égrève, France). Hepatocytes, monocytes, and hepatic stellate cells were conditioned for a minimum of 2 weeks in MammoCult® basal medium (StemCell) before use to sensitize them to the cell educating technology.
Educated macrophages and dendritic cells were prepared for each healthy donor in OneSmartDiff medium (PredictCan Biotechnologies, Grabels, France), a customizable person-dependent medium. OneSmartDiff medium is a defined medium on the basis of MammoCult® basal medium containing a broad-spectrum antibiotic supplemented with serum prepared from each individual. Absence of mycoplasma contamination was verified using MycoAlert® Mycoplasma Detection Kit from Lonza (Saint-Beauzire, France).
Primary human hepatocytes were purchased from Lonza.
Troglitazone, rosiglitazone, and rifampicin were purchased from CliniSciences (Nanterre, France).
Educated spheroid preparation and treatments
Educated spheroids were generated from a co-culture of HepG2 and TWNT-1 in ultra-low attachment plates, using blood from healthy donors. The system is supplemented with educated monocytes and cultured for 3 days before being treated for 3 days with TGZ with concentrations up to 100 × Cmax. Cmax values were obtained from clinical pharmacokinetic studies (TGZ-Cmax = 6.39 µM; RGZ-Cmax = 1.04 µM; Rifampicin-Cmax = 6.08 µM). Importantly, educated spheroids, educated macrophages, and dendritic cells were prepared from the same donor, and co-cultured together. The cell viability was then measured after 3 days of treatment. We generated an inhibitory dose–response curve fit with constrains (top = 100; bottom = 0).
For cell viability recovery experiment, educated spheroids were treated simultaneously with TGZ at Cmax and with recombinant human IL-12 at concentrations ranging from 0 to 1000 ng/ml.
For qPCR analysis, individual-centric spheroids and primary human hepatocytes were treated for 3 to 6 days with troglitazone, rosiglitazone, and rifampicin at Cmax.
Cell viability
Cell viability was measured using CellTiterGlo (Promega, Charbonnières-les-Bains, France) according to the manufacturer’s instructions.
Quantitative PCR
RNA extraction was performed using Arcturus® PicoPure® RNA Isolation Kit (Applied Biosystems™ by Life technologies™) and RNeasy Mini Kit (QIAGEN) according to manufacturer’s instructions. Reverse transcription was performed using OneScript® RT Mix for qPCR w/gDNAOut (Ozyme) followed by an amplification with ONEGreen® FAST qPCR Premix (Ozyme) according to manufacturer’s instructions. Primers (IL-10, IL-23, IL-12a, TNF-α, TGF-β, IL-6, IL-1B, CYP1A1, CYP2B6, CYP2C9, CYP2D6, CYP3A4, MAOA, CES1, UGT1A1, UGT2A3, UGT2B7, GSTP1, PXR, CAR, CEBPα, HNF4α, and GusB) were purchased from BIORAD. Quantitative PCR was performed on an IVDR-compliant thermal cycler (Thermo Fisher Scientific, QuantStudio 5 Dx Real-Time PCR System). All CTs were collected and the ∆CT were calculated by subtracting to GusB (housekeeping gene) CT. Relative expression to GusB for each gene was calculated using the formula RE = 2−∆CT.
Graphs and statistics
Plots and statistics were generated using GraphPad Prism v9 (Dotmatics, San Diego, CA) otherwise Excel (Microsoft Office 364).
All authors had access to the study data and had reviewed and approved the final manuscript.
Results
Interindividual heterogeneity of expression of xenobiotic receptors, transcription factors, and of induction of drug-metabolizing enzymes
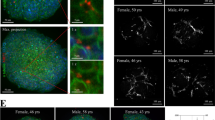
To confirm that individual-centric spheroids generated using blood sera from different subjects reproduced the biodiversity between individuals, we analyzed the basal expression of the xenobiotic receptors PXR and CAR in individual-centric spheroids from 2 males and 2 females with the age ranging from 31 to 37-year-old and compared to the expression in primary human hepatocytes (PHHs) prepared from 3 independent donors (Fig. 1a). As expected, we found a variation in the expression of PXR and CAR between donors in PHHs as well as in individual-centric spheroids. As a group, PHHs showed a slightly more expression of NR1I2/PXR and of NR1I3/CAR as compared to the group of individual-centric spheroids. We noticed that NR1I2/PXR expression was reduced in individual-centric spheroids at day 6. However, NR1I2/PXR expression was found to increase upon treatment with troglitazone at Cmax (Fig. 1b). Similarly, we noted that the expression of the transcription factors HNF4α and CEBPα, that regulates liver-specific genes that are involved in drug metabolism, was as high in individual-centric spheroids as in PHHs and was maintained overtime (Fig. 1a). All together, these data suggest that PHHs and individual-centric spheroids can both sense toxic byproducts and exogenous drugs to induce phases 1 and 2 drug-metabolizing enzymes (DEMs).
No clear correlation between TGZ-mediated induction of drug-metabolizing enzymes and iDILI risk occurrence. Individual-centric spheroids were prepared using depleted serum from 2 males and 2 females with the age ranging from 31 to 37-year-old. Primary human hepatocytes were from 3 independent donors. All cells were treated for up to 6 days with troglitazone and rifampicin at Cmax. A Basal expression of xenobiotic receptors PXR and CAR in primary human hepatocytes and in individual-centric spheroid models. Results are shown as relative expression to GusB gene. B Induction of NR1I2/PXR upon TGZ treatment. C Differential induction of phase 1 and phase 2 drug-metabolizing enzymes in primary human hepatocytes. D Differential induction of phase 1 and phase 2 drug-metabolizing enzymes in individual-centric spheroids. Individual-centric spheroids were prepared using depleted serum from 6 males and 2 females with the age ranging from 31 to 44-year old. The expression of the genes was quantified by qPCR. Results are shown in a heatmap as mean fold change to untreated. Each condition was analyzed in triplicate. E Individual-centric spheroid models were generated from 8 individuals who showed TGZ-mediated induction of CYP3A4 or not. They are then treated with troglitazone at concentrations up to 100 × Cmax. Cell viability was measured, and non-linear curve fits were drawn. No iDILI risk (i.e. cell death before Cmax value) was detected despite the induction of CYP3A4
We next analyzed the induction of phases 1 and 2 DEMs in PHHs and in individual-centric spheroids. Figure 1c shows the induction of phase 1 and phase 2 DEMs in PHHs in response to troglitazone (TGZ) and rifampicin at Cmax. We found that the type of DEMs and the magnitude of induction were donor dependent. Among all phase 1 DEMs, CYP3A4 was the most strongly induced by TGZ and rifampicin while the other phase 1 DEMs were only mildly or weakly induced. Most phase 2 DEMs were only weakly induced by both drugs in PHHs. We then measured the induction of phase 1 and phase 2 DEMs in individual-centric spheroids prepared from 8 subjects (6 males and 2 females). As expected, we found a heterogeneity in the induction and in the magnitude of expression in individual-centric spheroids (Fig. 1d). Among the phase 1 DEMs, CYP2B6, CYP2C9, and CYP2D6, that contribute to the metabolism of 10%, 20%, and 20% of commonly used drugs, respectively, were the most highly induced in an individual dependent manner (Fig. 1d). Furthermore, we observed an overall weaker induction of phase 2 DEMs (UGT1A1, UGT2A3, UGT2B7, and GSTP1) than phase 1 DEMs in both individual-centric spheroids and PHHs confirming a detoxifying capacity of both systems. Interestingly, we noticed that contrary to PHHs, CYP3A4 was not systematically induced by TGZ nor rifampicin in individual-centric spheroids. For instance, among 8 individuals only a 35-year-old male, a 31-year-old male, a 32-year-old female, and a 37-year-old female have shown an induction of CYP3A4 in response to TGZ (Fig. 1d). The metabolism of TGZ was reported as more dependent on CYP3A4 than other CYPs, and its reactive metabolites are responsible of liver toxicity. Thus, all 3 PHHs donors who showed TGZ-mediated CYP3A4 induction should be at iDILI risk. This statement contradicts the feature of iDILI which should be a rare event. Similarly, the 35-year-old male, the 31-year-old male, the 32-year-old female, and the 37-year-old female who showed induction of CYP3A4 by TGZ should be at risk for DILI as well. However, we couldn’t confirm that those 4 individuals have more iDILI risk than the other 4 individuals with no induction of CYP3A4 in our individual-centric spheroid models (Fig. 1e). To that point, we don’t have any direct evidence that TGZ-mediated iDILI risk is associated to CYP3A4 and our data suggest that it might involve another mechanism probably linked to immune response as there are growing evidence that most iDILI is immune mediated.
Troglitazone-mediated suppression of IL-12 expression by macrophages and dendritic cells is associated with iDILI risk occurrence
We first generated individual-centric spheroids from 10 subjects (5 males, age = 40.0 ± 5.2-year-old; 5 females, age = 42.2 ± 5.4-year-old) and then treated them with TGZ or with RGZ, its non-iDILI partner, with concentrations up to 100 × Cmax. We found that only a 44-year-old male out of a group of 10 individuals, showed cell death before Cmax suggesting an iDILI risk (Fig. 2a). RGZ did not show any cell death even up to 100 × Cmax. To explore the immune-related hypothesis of TGZ-mediated iDILI, we analyzed the direct effect of TGZ on activated macrophages and dendritic cells from a cohort of 10 individuals. For that, monocytes were cultured in the absence of hepatocytes and of hepatic stellate cells, in the tailored OneSmartDiff medium to trigger their differentiation into activated macrophages and DCs in a subject dependent manner. We then treated the monocyte-derived macrophages and DCs with TGZ or RGZ at Cmax and analyzed a panel of cytokines including IL-10, IL-23, IL-12a, TNF-α, TGF-β, IL-6, and IL-1B (Fig. 2b). We found that these cytokines were differentially induced by TGZ and RGZ in a donor dependent manner. However, only IL-12a transcript was downregulated by TGZ in the 44-year-old male who showed an iDILI risk. For the other cytokines, we couldn’t identify any expression profile that might be associated to iDILI risk. These results suggest that the downregulation of IL-12 by TGZ in macrophages and dendritic cells might contribute to iDILI occurrence in the 44-year-old male.
Troglitazone-mediated suppression of IL-12 expression by macrophages and dendritic cells is associated with iDILI risk in a 44-year-old male. A Individual-centric spheroid models were generated from a cohort of 10 individuals and then treated with troglitazone or with its non-iDILI partner rosiglitazone at concentrations up to 100 × Cmax. Cell viability was measured, and non-linear curve fits were drawn. Only a 44-year-old male showed an iDILI risk. B Educated macrophages and dendritic cells were derived from monocytes cultured in OneSmartDiff medium containing depleted serum from each donor. Quantitative PCR was performed to measure the expression of a panel of cytokines. Results are shown in a heatmap as mean fold change to untreated. Each condition was analyzed in triplicate. C Educated spheroids containing hepatocytes, hepatic stellate cells, macrophages, and dendritic cells were prepared using depleted serum from a 44-year-old male with iDILI risk. Spheroids were then treated with troglitazone at Cmax alone or in combination with increasing doses of recombinant human IL-12. Cell viability was measured using CellTiterGlo and results are shown as mean percentage of viable cells. Each condition was performed in triplicate
To confirm that hypothesis, we generated educated spheroids using depleted serum from the 44-year-old male and then treated them with TGZ alone at Cmax or in combination with increasing amounts of recombinant IL-12 (Fig. 2c). As expected, we found that combinatory treatment with IL-12 reduced cell death dose-dependently with a complete recovery at 10 ng/ml confirming the role of IL-12 in TGZ-mediated iDILI risk in the 44-year-old male.
To analyze whether this suppression of IL-12 expression by TGZ is a general mechanism linked to iDILI risk or it is specific to the 44-year-old male, we identified another individual, a 31-year-old female, who showed iDILI risk upon TGZ treatment (Fig. 3a). We showed that treatment with TGZ caused a stronger reduction of IL-12a expression by macrophages and dendritic cells in the 31-year-old female than in the 44-year-old male (Fig. 3b). Consequently, we demonstrated that a higher amount of recombinant IL-12 was required to rescue iDILI risk in the 31-year-old female (1000 ng/ml) than in the 44-year-old male (10 ng/ml) (Fig. 3c). Our data suggest that TGZ-mediated suppression of IL-12 expression by macrophages and dendritic cells might be a general mechanism of iDILI occurrence.
Troglitazone-mediated suppression of IL-12 expression is also associated to iDILI risk in the 31-year-old female. A Educated spheroids were prepared using depleted serum from a 31-year-old female and then treated with TGZ with concentrations up to 100 × Cmax. Cell viability was measured, and a non-linear curve fit was drawn. Cell viability dropped before Cmax confirming an iDILI risk in the 31-year-old female. B Activated macrophages and dendritic cells were prepared with the OneSmartDiff medium containing the depleted serum from the 31-year-old female, and then treated with TGZ at Cmax. The expression of IL-12a was monitored by qPCR. Results are shown as fold change to untreated. C Individual-centric spheroids were reconstructed with autologous hepatocytes, hepatic stellate cells, macrophages, and dendritic cells, both prepared with the depleted serum from the 31-year-old female. Spheroids were then treated with TGZ alone at Cmax, or in combination with increasing doses of recombinant IL-12. Cell viability was measured using CellTiterGlo and results are shown as mean percentage of viable cells. Each condition was performed in triplicate
All together our data demonstrated that the initiating-mechanism of iDILI could be uncovered using individual-centric spheroid models as the system allows to separate autologous cell populations to assess, for example, a direct effect of the drug on immune cells. We, therefore, propose a multi-steps workflow consisting of, first, identifying the subject at risk for iDILI in a cohort of individuals, second, deciphering the immune-mediated drug-induced iDILI mechanism using for instance only autologous immune cells, and third, confirming the mechanism in a full microenvironment (i.e., individual-centric spheroids containing autologous hepatocytes, hepatic stellate cells, activated macrophages, and DCs) (Fig. 4). To our best knowledge, this is the first individual-centric system with a high flexibility that allows to study the effect of a drug on separate cell populations prior a full reconstruction of the microenvironment for target validation.
Example of workflow for the analysis of the initiating-mechanism of iDILI using individual-centric spheroid models. In step 1, the idiosyncratic DILI risk is identified in a cohort of subjects. The analysis of the direct contribution of immune cells in iDILI risk is done in step 2 enabling the identification of a potential driver. In step 3, the iDILI driver is confirmed in a fully reconstructed spheroid system
Discussion
The initiating-mechanism of iDILI is challenging to identify as the phenomenon is unpredictable and rare. Until recently, there is no preclinical models that can predict iDILI risk in a cohort of subjects at early stage of drug development. Indeed, attempts to use preclinical models including primary liver cells, organoids, or iPSCs, to detect iDILI risk at early stage of drug development are unsatisfactory as these systems often spot DILI at unphysiological concentrations of the drug. Moreover, their complexity makes impossible to separate different cell populations for the analysis of the direct effect of the drug on each population prior a reconstruction of a full microenvironment to confirm the mechanism of iDILI. The individual-centric spheroid model was the first reported as an in vitro model that can capture immune-mediated iDILI risk (Roux et al. 2024). In the present work, we highlighted the flexibility of this model separating the immune cell population from the hepatic parenchymal and non-parenchymal cells to uncover the initiating-mechanism of TGZ-mediated iDILI. Our system opens perspectives for safer lead compound selection during drug development and for strategies to mitigate iDILI in a personalized approach.
Cell lines such as HepG2 are often neglected in favor of primary liver cells in drug-induced liver injury analysis, because they have an overall lower expression of DMEs. Cytochrome P450 enzymes metabolize about 70% of the drugs. Although CYP3A4/5 is highly expressed in hepatocytes and is involved in the metabolism of 30% of the drugs, the other members of CYP family such as CYP2D6, CYP2C9, and CYP2B6 contribute also substantially to 20%, 13%, and 7% of the process, respectively (Zhao et al. 2021). Moreover, the same drug could be bio-transformed by multiple CYPs(Preissner et al. 2013) suggesting a possible compensatory mechanism when one CYP is under represented and thus, studying drug biotransformation and DILI risk, one should not consider only CYP3A4 but also other CYP family members. Our data clearly show that CYP3A4 was predominantly expressed in all donors upon TGZ treatment in primary human hepatocytes. Considering the hypothesis that TGZ-mediated iDILI is caused by its metabolism by CYP3A4, one should, therefore, observe a DILI risk in all 3 donors. This contradicts the iDILI feature as it should be a rare event. Furthermore, previous studies using primary human hepatocytes to assess TGZ-mediated DILI have shown that cell death occurred in all donors at concentrations above Cmax suggesting that primary hepatocytes cannot detect drug-induced liver toxicity at therapeutic dose despite the elevated expression of CYP3A4 (Vanden Heuvel et al. 2018). Our observation that high CYP3A4 expression is not directly linked to iDILI occurrence, suggests that the contribution of other CYP family members should be considered when one analyzes how safe is a molecule and for drug-drug interaction studies. Further analyses are needed to clarify the role of each CYP family members and how they act together to trigger iDILI.
Idiosyncratic DILI is generally believed to be mediated by the adaptive immune response. However, this adaptive immune activation requires first an innate immune response to trigger cytokines production for T cells proliferation (Carpenter and O'Neill 2024). The activation of the innate immune response is likely caused by reactive metabolites produced by DEMs or by diverse forms of cell stress such as mitochondrial injury or blockade of bile salt export pump (Chen et al. 2023; Fiorucci et al. 2018). Another alternative way to stimulate the initiation of the innate immune response is a direct effect of the drug on immune cells. This hypothesis is generally not investigated as there is no possibility to separate autologous cell populations in a complex in vitro model. TGZ is a selective agonist of the peroxisome proliferator-activated receptor γ (PPARγ) that is expressed in macrophages and dendritic cells. Studies have reported that the activation of PPARγ can negatively modulate the production of inflammatory cytokines (Wang et al. 2017; Yang et al. 2008). Our data showing that TGZ suppressed IL-12 expression by macrophages and DCs in the 44-year-old male and in the 31-year-old female suggest that this event could be an initiating mechanism of the activation of the innate immune response. Moreover, we demonstrated that combinatory treatment with TGZ and IL-12 suppressed cell death confirming that IL-12 could have a protective role in TGZ-mediated iDILI. More studies are required to understand how PPARγ regulates IL-12 expression.
There is evidence that cytokines can protect from liver injury (Pratim Das and Medhi 2023). For instance, it has been reported that IL-24 protects mice from thioacetamide-induced liver injury via the inhibition of hepatic stellate cells activation (Wang et al. 2021). Similarly, IL-12 produced by Kuffer cells and DCs through the cGAS-STING pathway, was shown to protect mice from acetaminophen-mediated liver injury (Hildreth et al. 2023). Our findings that IL-12 mitigates TGZ-mediated iDILI in the 44-year-old male and the 31-year-old female are in line with those observations.
Idiosyncratic DILI is a multifactorial event. Its occurrence depends on the age, the sex, and the immune landscape of each individual. Thus, the mechanism can vary from person to person making that its prediction is challenging because there is no preclinical model with high predictivity. We have previously demonstrated that the individual-centric spheroid model is a valuable alternative to models that use primary cells to analyze DILI risk (Cherradi et al. 2023; Roux et al. 2024). Importantly, educated spheroids can identify iDILI risk at therapeutic doses based on non-genetic factors such as age and sex (Cherradi et al. 2023; Roux et al. 2024). In the present work, we showed that it is possible to exploit this model to analyze individualized immune landscape to uncover the iDILI initiating mechanism for each subject. Our results encourage case report studies of liver toxicity with a perspective of its use as a companion test to mitigate iDILI risk.
In summary, we present here data that support the use of individual-centric spheroid models to decipher the initiating mechanism of iDILI owning the flexibility of this model that allows to separate autologous cell populations for mechanistic studies prior reconstruction of the full model to validate the findings. Thus, the individual-centric spheroid model is a meaningful model to deconvolute drug-induced liver injury identified by other preclinical models including liver organoids or liver microtissues.
Data availability
The data generated in this study are not publicly available. Methods related to this study will be shared on reasonable request with permission of PredictCan Biotechnologies SAS.
Abbreviations
- iDILI:
-
Idiosyncratic drug-induced liver injury
- TGZ:
-
Troglitazone
- RGZ:
-
Rosiglitazone
- DC:
-
Dendritic cells
- DEMs:
-
Drug-metabolizing enzymes
References
Allison R, Guraka A, Shawa IT, Tripathi G, Moritz W, Kermanizadeh A (2023) Drug induced liver injury - a 2023 update. J Toxicol Environ Health B Crit Rev 26(8):442–467. https://doi.org/10.1080/10937404.2023.2261848
Carpenter S, O’Neill LAJ (2024) From periphery to center stage: 50 years of advancements in innate immunity. Cell 187(9):2030–2051. https://doi.org/10.1016/j.cell.2024.03.036
Chen S, Liao Z, Xu P (2023) Mitochondrial control of innate immune responses. Front Immunol 14:1166214. https://doi.org/10.3389/fimmu.2023.1166214
Cheng D, Chai J, Wang H, Fu L, Peng S, Ni X (2021) Hepatic macrophages: Key players in the development and progression of liver fibrosis. Liver Int 41(10):2279–2294. https://doi.org/10.1111/liv.14940
Cherradi S, Taulet N, Duong HT (2023) An original donor-dependent spheroid system for the prediction of idiosyncratic drug-induced liver injury risk. In Vitro Models 2(6):281–295. https://doi.org/10.1007/s44164-023-00057-w10.1007/s44164-023-00057-w
Chojkier M (2005) Troglitazone and liver injury: in search of answers. Hepatology 41(2):237–246. https://doi.org/10.1002/hep.20567
Fiorucci S, Biagioli M, Zampella A, Distrutti E (2018) Bile acids activated receptors regulate innate immunity. Front Immunol 9:1853
Fontana RJ, Bjornsson ES, Reddy R, Andrade RJ (2023) The evolving profile of idiosyncratic drug-induced liver injury. Clin Gastroenterol Hepatol 21(8):2088–2099. https://doi.org/10.1016/j.cgh.2022.12.040
Funk C, Ponelle C, Scheuermann G, Pantze M (2001) Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: in vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol Pharmacol 59(3):627–635
Haskins JR, Rowse P, Rahbari R, de la Iglesia FA (2001) Thiazolidinedione toxicity to isolated hepatocytes revealed by coherent multiprobe fluorescence microscopy and correlated with multiparameter flow cytometry of peripheral leukocytes. Arch Toxicol 75(7):425–438. https://doi.org/10.1007/s002040100251
Hildreth AD, Padilla ET, Tafti RY, Legala AR, O’Sullivan TE (2023) Sterile liver injury induces a protective tissue-resident cDC1-ILC1 circuit through cDC1-intrinsic cGAS-STING-dependent IL-12 production. Cell Rep 42(2):112141. https://doi.org/10.1016/j.celrep.2023.112141
Jee A, Sernoskie SC, Uetrecht J (2021) Idiosyncratic drug-induced liver injury: mechanistic and clinical challenges. Int J Mol Sci 22(6):2954. https://doi.org/10.3390/ijms22062954
Kenna JG, Uetrecht J (2018) Do in vitro assays predict drug candidate idiosyncratic drug-induced liver injury risk? Drug Metab Dispos 46(11):1658–1669. https://doi.org/10.1124/dmd.118.082719
Lendeckel U, Venz S, Wolke C (2022) Macrophages: shapes and functions. ChemTexts 8(2):12. https://doi.org/10.1007/s40828-022-00163-4
Mak A, Uetrecht J (2019) Involvement of CCL2/CCR2 macrophage recruitment in amodiaquine-induced liver injury. J Immunotoxicol 16(1):28–33. https://doi.org/10.1080/1547691X.2018.1516014
Mendez-Sanchez N, Cordova-Gallardo J, Barranco-Fragoso B, Eslam M (2021) Hepatic dendritic cells in the development and progression of metabolic steatohepatitis. Front Immunol 12:641240. https://doi.org/10.3389/fimmu.2021.641240
Padberg F, Hoper T, Henkel S, Driesch D, Luch A, Zellmer S (2021) Novel indirect co-culture of immortalised hepatocytes with monocyte derived macrophages is characterised by pro-inflammatory cytokine networks. Toxicol in Vitro 73:105134. https://doi.org/10.1016/j.tiv.2021.105134
Pratim Das P, Medhi S (2023) Role of inflammasomes and cytokines in immune dysfunction of liver cirrhosis. Cytokine 170:156347. https://doi.org/10.1016/j.cyto.2023.156347
Preissner SC, Hoffmann MF, Preissner R, Dunkel M, Gewiess A, Preissner S (2013) Polymorphic cytochrome P450 enzymes (CYPs) and their role in personalized therapy. PLoS ONE 8(12):e82562. https://doi.org/10.1371/journal.pone.0082562
Roux S, Cherradi S, Duong HT (2024) Exploiting the predictive power of educated spheroids to detect immune-mediated idiosyncratic drug-induced liver injury: the case of troglitazone. Front Pharmacol 15:1378371. https://doi.org/10.3389/fphar.2024.1378371
Segovia-Zafra A, Di Zeo-Sanchez DE, Lopez-Gomez C et al (2021) Preclinical models of idiosyncratic drug-induced liver injury (iDILI): Moving towards prediction. Acta Pharm Sin B 11(12):3685–3726. https://doi.org/10.1016/j.apsb.2021.11.013
Tirmenstein MA, Hu CX, Gales TL et al (2002) Effects of troglitazone on HepG2 viability and mitochondrial function. Toxicol Sci 69(1):131–138. https://doi.org/10.1093/toxsci/69.1.131
Uetrecht J (2019) Mechanisms of idiosyncratic drug-induced liver injury. Adv Pharmacol 85:133–163. https://doi.org/10.1016/bs.apha.2018.12.001
Vanden Heuvel JPM, S.; Granda, M. V.; Shef, B. Examination Of Drug-induced Liver Injury In upcyte Hepatocytes. In: Society of Toxicology (SOT), San Antonio, Texas, 2018.
Villar J, Coillard A, van Roessel C, Segura E (2023) Culture system allowing the simultaneous differentiation of human monocytes into dendritic cells and macrophages using M-CSF, IL-4, and TNF-alpha. Methods Mol Biol 2618:147–154. https://doi.org/10.1007/978-1-0716-2938-3_11
Wang D, Shi L, Xin W et al (2017) Activation of PPARgamma inhibits pro-inflammatory cytokines production by upregulation of miR-124 in vitro and in vivo. Biochem Biophys Res Commun 486(3):726–731. https://doi.org/10.1016/j.bbrc.2017.03.106
Wang HH, Huang JH, Sue MH et al (2021) Interleukin-24 protects against liver injury in mouse models. EBioMedicine 64:103213. https://doi.org/10.1016/j.ebiom.2021.103213
Yamashita YI, Imai K, Mima K et al (2017) Idiosyncratic drug-induced liver injury: a short review. Hepatol Commun 1(6):494–500. https://doi.org/10.1002/hep4.1064
Yang XY, Wang LH, Farrar WL (2008) A role for PPARgamma in the regulation of cytokines in immune cells and cancer. PPAR Res 2008:961753. https://doi.org/10.1155/2008/961753
Zhao M, Ma J, Li M et al (2021) Cytochrome P450 enzymes and drug metabolism in humans. Int J Mol Sci 22(23):12808. https://doi.org/10.3390/ijms222312808
Acknowledgements
This study was funded by the Region Occitanie and the French Government as part of the France 2030 Plan “Projets d’Innovation” DOS0222542/00 & DOS0222543/00, and by the French Government as part of the “Programme d’investissements d’avenir” (BFTE DOS0178148/00).
Author information
Authors and Affiliations
Contributions
S.R. performed all laboratory experiments. S.C. and H.T.D. analyzed the data. S.C., S.R., and H.T.D. conceived and planned experiments. S.C., S.R., and H.T.D. discussed results and contributed to the final article.
Corresponding author
Ethics declarations
Conflict of interest
S.C., S.R., and H.T.D. are employees of PredictCan Biotechnologies SAS. S.C. and H.T.D. are founders of PredictCan Biotechnologies SAS.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Roux, S., Cherradi, S. & Duong, H.T. Uncovering the mechanism of troglitazone-mediated idiosyncratic drug-induced liver injury with individual-centric models. Arch Toxicol (2024). https://doi.org/10.1007/s00204-024-03833-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00204-024-03833-z